Why Is A Gas Easier To Compress
Why Is A Gas Easier To Compress - Web the simple answer: Web why are gases easy to compress? What happens to gas particles when a gas is compressed. • fluids take the form of the container. We turn science into easily understood explanations for why gases are easy. Click the card to flip 👆. A.) its volume increases more under pressure than an equal volume of liquid does. Web why is a gas easier to compress than a liquid or solid? They typically contain 10 liters of gas (more specifically:. A gas takes the shape and size. The compression process involves a series of reciprocating compressors strategically placed. • a substance that can flow. They typically contain 10 liters of gas (more specifically:. A gas takes the shape and size. • fluids take the form of the container. Gases are compressible because most of the volume of a gas is. We turn science into easily understood explanations for why gases are easy. Web why is a gas easy to compress? This space allows us to put pressure on gas, and force it in a smaller container. Web why are gases easy to compress? Size of the gas particles is small. At room temperature and standard. Gases will compress more easily than solids or liquids because there is so much space between the gas molecules. They typically contain 10 liters of gas (more specifically:. Web study with quizlet and memorize flashcards containing terms like why is a gas easier to compress than a liquid. Web why is gas easier to compress than a liquid or a solid? Web study with quizlet and memorize flashcards containing terms like why is a gas easier to compress than a liquid or a solid?, why does the pressure inside a container of gas. • a substance that can flow. Web why is a gas easier to compress than. List three factors that can affect gas pressure. Web why is gas easier to compress than a liquid or a solid? What happens to gas particles when a gas is compressed. Web compressed gas is easier to transport with reduced volume and lower energy loss. B.) its volume increases more. B.) its volume increases more. A.) its volume increases more under pressure than an equal volume of liquid does. Web pressure is a function of the gas molecules which engaged in elastic collisions with themselves, and with the sides of the container. Web gases are compressible because most of the volume of a gas is composed of the large amounts. Web 1) why is a gas easier to compress than a liquid or a solid? Web gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles. Its volume increases more under pressure than an equal volume of solid does b. Web compressed gas is easier to. Its volume increases more under pressure than an equal volume of solid does b. Web the simple answer: At room temperature and standard. Web compressed gas is easier to transport with reduced volume and lower energy loss. The compression factor increases) but it also depends on temperature. Gases are easily compressed because of the space between the particles in a gas. Web pressure is a function of the gas molecules which engaged in elastic collisions with themselves, and with the sides of the container. • fluids take the form of the container. Its volume increases more under pressure than an equal volume of liquid does. And thus. Its volume increases more under. • a substance that can flow. Its volume increases more under pressure than an equal volume of solid does b. Gases are easily compressed because of the space between the particles in a gas. What happens to gas particles when a gas is compressed. What happens to gas particles when a gas is compressed. Web why are gases easy to compress? The compression factor increases) but it also depends on temperature. Web pressure is a function of the gas molecules which engaged in elastic collisions with themselves, and with the sides of the container. We turn science into easily understood explanations for why gases are easy. Web 1) why is a gas easier to compress than a liquid or a solid? Size of the gas particles is small. Web gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles. • fluids take the form of the container. Web thus if it has a higher pressure and smaller volume, it typically is harder to compress (i.e. Take a scuba tank for instance: Most of the volume was. • liquids (difficult to compress) • gases (easy to compress) • basic properties: Its volume increases more under pressure than an equal volume of solid does b. List three factors that can affect gas pressure. A gas takes the shape and size.
Activity to show that gases can be compressed more easily than liquids
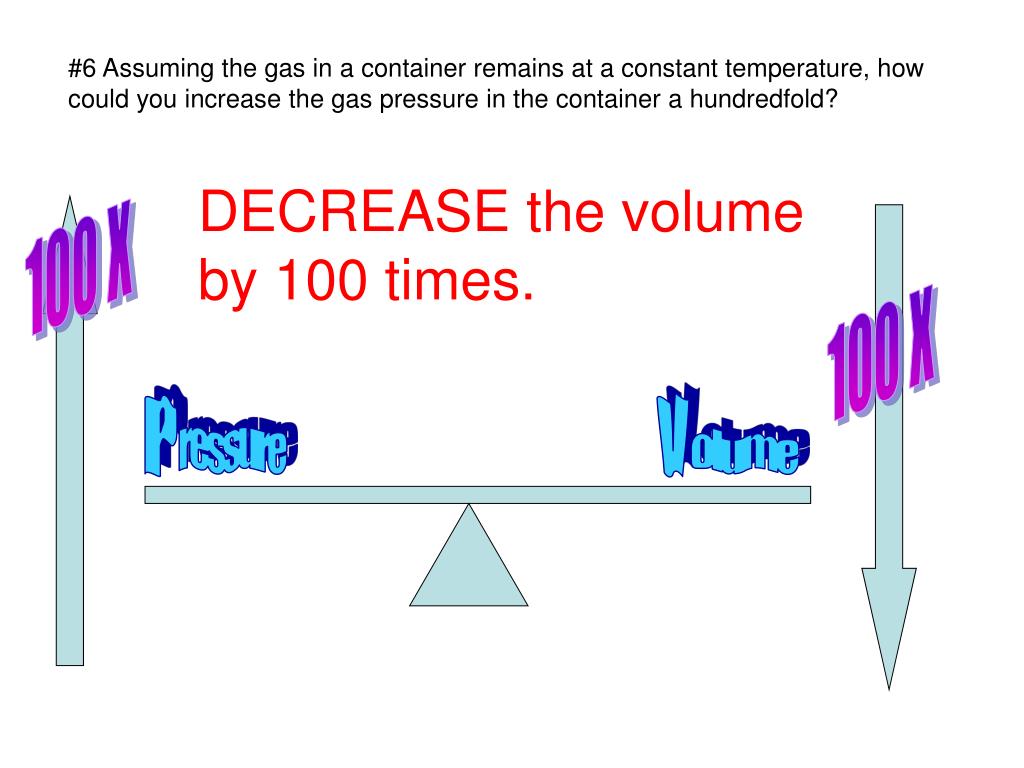
PPT Chapter 14.1 1 Key Concept Why is a gas easy to compress

PPT Chapter 14.1 1 Key Concept Why is a gas easy to compress

Gas can compress YouTube

PPT Chapter 14.1 1 Key Concept Why is a gas easy to compress

PPT Unit 11 Review PowerPoint Presentation, free download ID6902102

Why Can Gases Be Compressed CANZI

PPT Chapter 14 “The Behavior of Gases” PowerPoint Presentation, free

Why do pressure and temperature increase during the compression of a
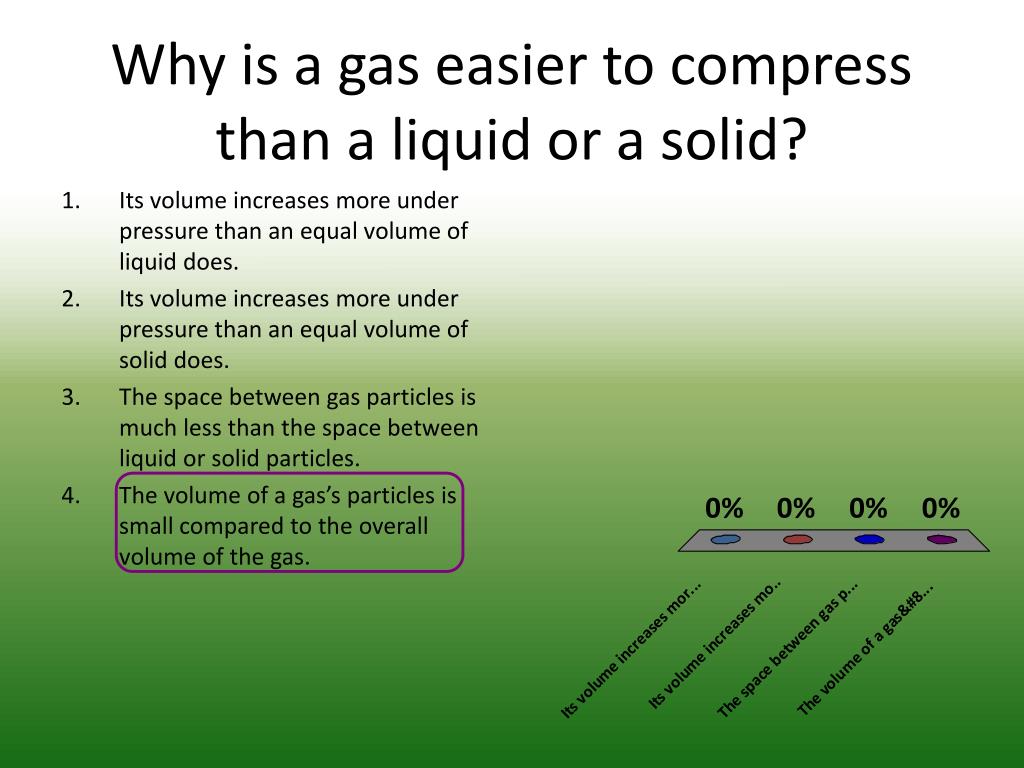
Why Are Gases Easy To Compress
Web Why Is A Gas Easy To Compress?
Gases Will Compress More Easily Than Solids Or Liquids Because There Is So Much Space Between The Gas Molecules.
Web Why Is A Gas Easier To Compress Than A Liquid Or A Solid?
And Thus For A Gas, Compression Of A Given Volume.
Related Post: