Quantum Mechanical Model Drawing
Quantum Mechanical Model Drawing - How did scientists figure out the structure of atoms without looking at them? Energy level and transition of electrons. The quantum mechanical model of the atom. Quantum mechanics and the atom. Whether you have laptops, ipads, chromebooks, or byod, your favorite phet sims are always right at your fingertips.become part of our mission today, and transform the learning experiences of students everywhere! Web quantum theory has some mathematical development, often referred to as quantum mechanics, that offers explanations for the behavior of electrons inside the electron clouds of atoms. Quantum mechanics and the atom. Web inserting n=2, l=0, and m=0 into the general wave function gives us the wave function for the 2s orbital (eq. The current view of atomic structure is that electrons exist in a cloud surrounding the nucleus, rather than in fixed orbits. By the end of this section, you will be able to: What does it mean that the energy of an electron is “quantized”? Web quantum mechanics is based on schrödinger’s wave equation and its solution. Web inserting n=2, l=0, and m=0 into the general wave function gives us the wave function for the 2s orbital (eq. As a result, this model is based on probability rather than certainty. We also explain. How did scientists figure out the structure of atoms without looking at them? To do so required the. Check how the prediction of the model matches the experimental results. We also explain how knowing the arrangement of electrons in an atom enables chemists to predict and explain the chemistry of an element. Draw the orbital shapes for the following orbitals. Web inserting n=2, l=0, and m=0 into the general wave function gives us the wave function for the 2s orbital (eq. It provides a neat and familiar picture of electrons orbiting a central nucleus like planets around the sun. Try out different models by shooting light at the atom. E ( n) = − 1 n 2 ⋅ 13.6 ev.. Quantum mechanics and the atom. The current view of atomic structure is that electrons exist in a cloud surrounding the nucleus, rather than in fixed orbits. The periodic table, electron shells, and orbitals. Web quantum mechanical model of atom structure. Web this model, which is the basis of the modern understanding of the atom, is known as the quantum mechanical. Web by converting our sims to html5, we make them seamlessly available across platforms and devices. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web quantum theory has some mathematical development, often referred to as quantum mechanics, that offers explanations for the behavior of electrons inside the. As a result, this model is based on probability rather than certainty. The periodic table, electron shells, and orbitals. Web quantum theory has some mathematical development, often referred to as quantum mechanics, that offers explanations for the behavior of electrons inside the electron clouds of atoms. Continuum modeling approaches and solution approaches. Whether you have laptops, ipads, chromebooks, or byod,. Equation 1.2.12 wave function for the 2s orbital. Web quantum mechanical model of atom structure. Web inserting n=2, l=0, and m=0 into the general wave function gives us the wave function for the 2s orbital (eq. To do so required the. How did scientists figure out the structure of atoms without looking at them? Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Continuum modeling approaches and solution approaches. Bohr's model calculated the following energies for an electron in the shell, n. Web this model, which is the basis of the modern understanding of the atom, is known as the quantum mechanical. Thus, the 2s orbital is also a spherical orbital. E ( n) = − 1 n 2 ⋅ 13.6 ev. Atomic theories\' the quantum mechanical model of the atom. Web the libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc. We also acknowledge previous national science foundation support under. Like in the case of the 1s orbital, the angular parts of the wave function are only simple numbers. Web quantum theory has some mathematical development, often referred to as quantum mechanics, that offers explanations for the behavior of electrons inside the electron clouds of atoms. Quantum numbers for the first. Understand the general idea of the quantum mechanical description of electrons in orbitals. Web the libretexts libraries are powered by nice cxone expert and are supported by the department of education open textbook pilot project, the uc davis office of the provost, the uc davis library, the california state university affordable learning solutions program, and merlot. Like in the case of the 1s orbital, the angular parts of the wave function are only simple numbers. To do so required the. Continuum modeling approaches and solution approaches. The bohr planetary model of the atom is often what sticks in students’ minds. The quantum mechanical model of the atom. To do so required the. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, n. Web this model, which is the basis of the modern understanding of the atom, is known as the quantum mechanical or wave mechanical model. Quantum mechanics and the atom. The quantum mechanical model of the atom. How did scientists figure out the structure of atoms without looking at them? Check how the prediction of the model matches the experimental results. Web by converting our sims to html5, we make them seamlessly available across platforms and devices.
The Quantum Mechanical Model Overview, Definition & Discovery

PPT The Quantum Model PowerPoint Presentation, free download ID2574252

Chapter 7 The QuantumMechanical Model of the Atom
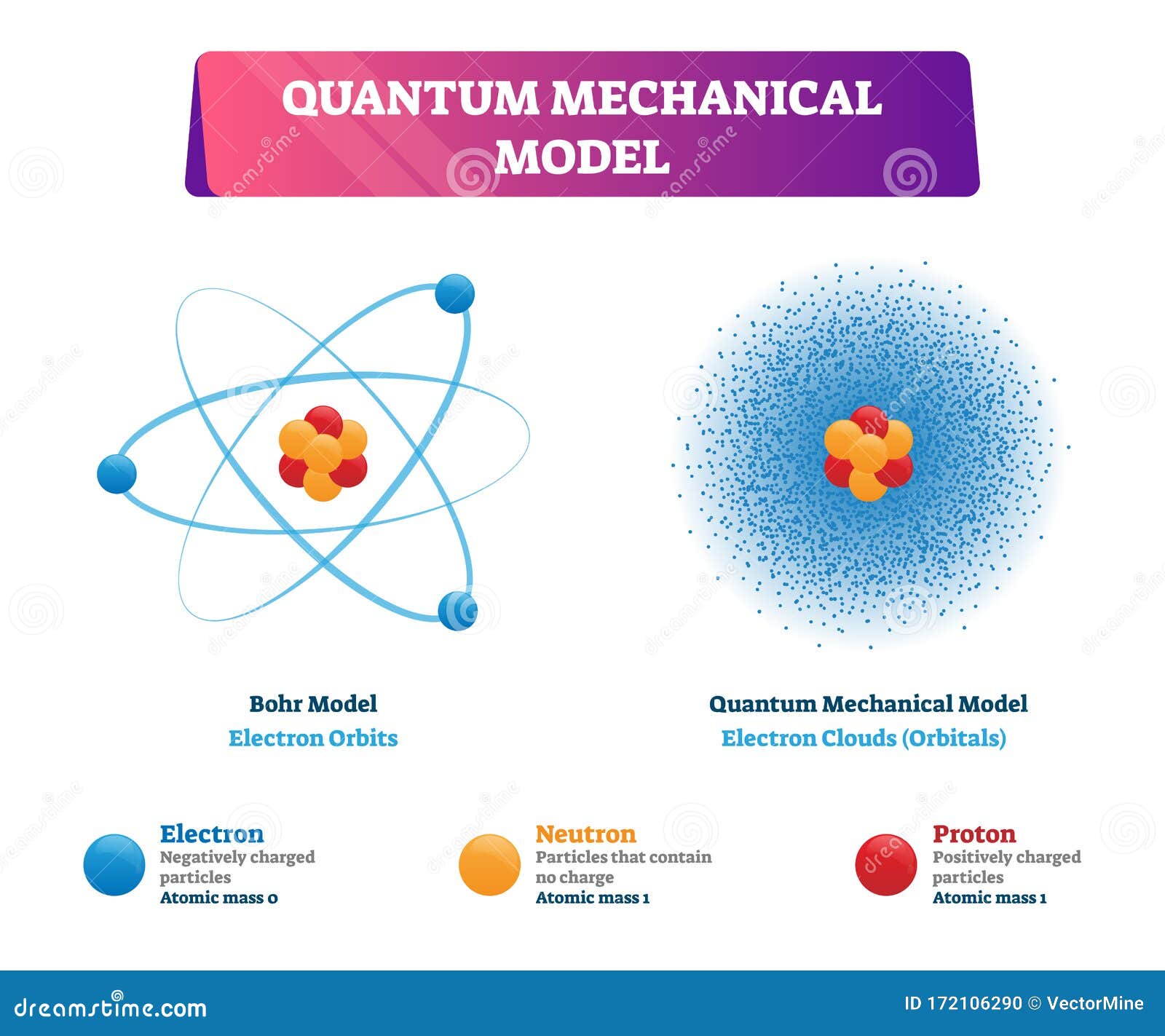
Quantum Atomic Model

PPT Quantum Mechanical Model PowerPoint Presentation, free download

Physics Quantum Mechanics, Particles, Waves Britannica
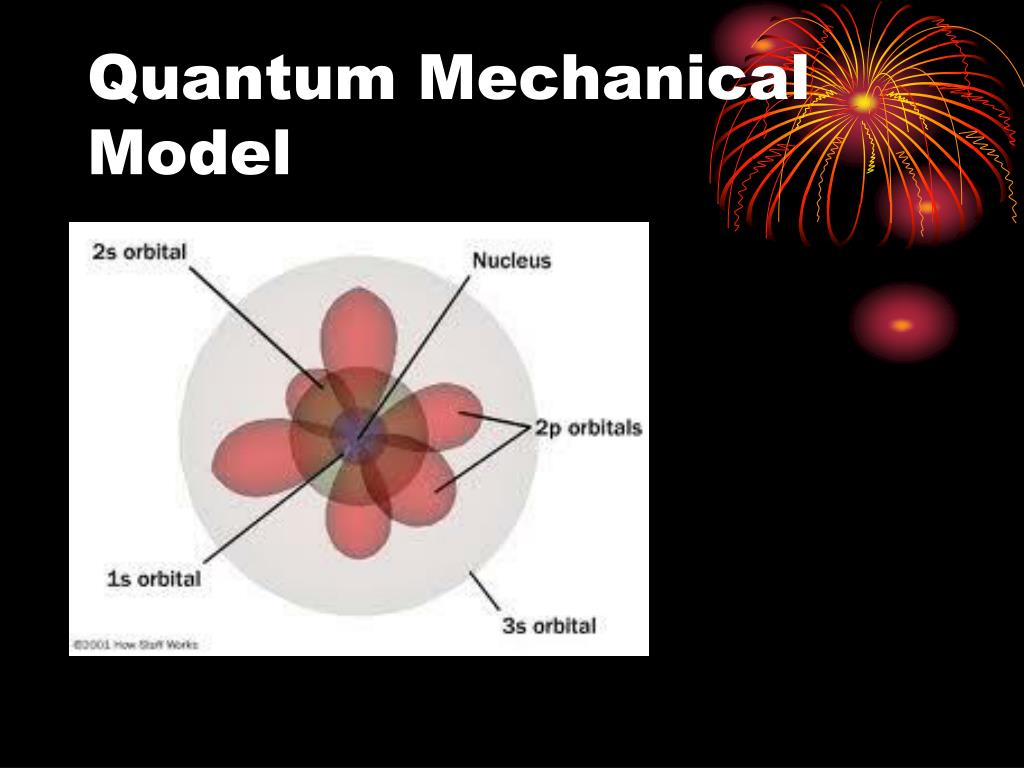
PPT Quantum Mechanical Model of the Atom PowerPoint Presentation

Introduction to the Quantum Mechanical Model of the Atom (PART 1) YouTube

The Quantum Model Quantum Computing

Hydrogen Atom Quantum Mechanical Model Of Hydrogen Atom
Draw The Orbital Shapes For The Following Orbitals (Label All Three Axes):
The Current View Of Atomic Structure Is That Electrons Exist In A Cloud Surrounding The Nucleus, Rather Than In Fixed Orbits.
E ( N) = − 1 N 2 ⋅ 13.6 Ev.
Web In Contrast To Bohr’s Model, However, Which Allowed Only One Orbit For Each Energy Level, Quantum Mechanics Predicts That There Are 4 Orbitals With Different Electron Density Distributions In The N = 2 Principal Shell (One 2S And Three 2P Orbitals), 9 In The N = 3 Principal Shell, And 16 In The N = 4 Principal Shell.
Related Post: