Particle Drawing Of Mgcl2
Particle Drawing Of Mgcl2 - Count the number of electrons used in the mgcl 2 structure up to the outermost valence shell. To be consistent with the law of conservation of mass, the diagram should depict the same numbers and types of atoms on each side of the reaction arrow. The repeating part, c, of the infinite chain in mgal2dl8. Web the lewis structure of mgcl2 is therefore. (3 pts) draw one fully solvated potassium ion and one fully solvated chloride ion in an aqueous solution. These valence electrons are the building blocks of the structure alongside the individual atoms. These salts are colorless or white solids that are highly soluble in water. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. First, we must calculate the valence electrons available to us. Gas phase ion energetics data. And interatomic distances are given in table 6. Web at the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Web this in turn will create an induced dipole in neighboring molecules. 2.32 g/cm 3 (anhydrous) boiling point of magnesium chloride. 95.211 g/mol (anhydrous) the density of magnesium chloride. And interatomic distances are given in table 6. 2.32 g/cm 3 (anhydrous) boiling point of magnesium chloride. A given chemical reaction can be represented using a particulate diagram, in which the reaction mixture is depicted both before the reaction occurs and after the reaction has proceeded completely as possible. It has a boiling point of 1685 k and a density. (3 pts) draw one fully solvated potassium ion and one fully solvated chloride ion in an aqueous solution. I am available to help you find solutions to you. These valence electrons are the building blocks of the structure alongside the individual atoms. Web this in turn will create an induced dipole in neighboring molecules. > mgcl_2 is an ionic compound. Web mgcl2 valence electrons. We have calculated the number of valence electrons in. To be consistent with the law of conservation of mass, the before reaction and after reaction visualizations should each. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. 95.211 g/mol (anhydrous) the density of magnesium chloride. A given chemical reaction can be represented using a particulate diagram, in which the reaction mixture is depicted both before the reaction occurs and after the reaction has proceeded completely as possible. Web mgcl2 is an ionic halide salt consisting of magnesium and chlorine elements. The electron configuration of mg is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Here’s the best. These compounds and their solutions, both of which occur in nature, have a variety of practical uses. Web mgcl2 valence electrons. The electron configuration of mg is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. To be consistent with the law of conservation of mass, the. (from www.superteachertools.net) the compound is held together by electrostatic attractions between the positively charged magnesium ion and the negatively charged chloride ions. The right drawing is turned 90° compared to the left and lacks the three upper and the three lower chlorine atoms for clarity. Web a balanced chemical equation can be visualized using a particulate diagram, in which each. It has a boiling point of 1685 k and a density of 2.32 g/cc. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. In its anhydrous form, it consists of about 25.5% mg by mass and has a molar mass of about 95.211 g/mol. Gas phase ion energetics data. Web how to draw. Gas phase ion energetics data. > mgcl_2 is an ionic compound. Web the lewis structure of mgcl2 is therefore. The electron configuration of mg is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. It forms hydrates mgcl2·nh2o, where n can range from 1 to 12. I am available to help you find solutions to you. These salts are colorless or white solids that are highly soluble in water. > mgcl_2 is an ionic compound. The repeating part, c, of the infinite chain in mgal2dl8. (from www.superteachertools.net) the compound is held together by electrostatic attractions between the positively charged magnesium ion and the negatively charged chloride. 95.211 g/mol (anhydrous) the density of magnesium chloride. Web clearly represent any differences in how each ion is solvated. We have calculated the number of valence electrons in. I am available to help you find solutions to you. However, as mentioned, these dipoles are temporary since the electrons can just as easily shift to a new position in the particle. It forms hydrates mgcl2·nh2o, where n can range from 1 to 12. Here’s the best way to solve it. Web a balanced chemical equation can be visualized using a particulate diagram, in which each of the atoms involved in the reaction is represented using a circle or a sphere. In its anhydrous form, it consists of about 25.5% mg by mass and has a molar mass of about 95.211 g/mol. Web mgcl2 is an ionic halide salt consisting of magnesium and chlorine elements. Web how to draw the lewis dot structure for mgcl2?a quick introduction about me, salutations, my name is delphi. 2.32 g/cm 3 (anhydrous) boiling point of magnesium chloride. Web mgcl2 valence electrons. Web refer to the video. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. Web this in turn will create an induced dipole in neighboring molecules.
Mgcl2 Lewis Structure

draw atomic structure of mgcl2 Brainly.in

MgCl2 Molecular Geometry Science Education and Tutorials
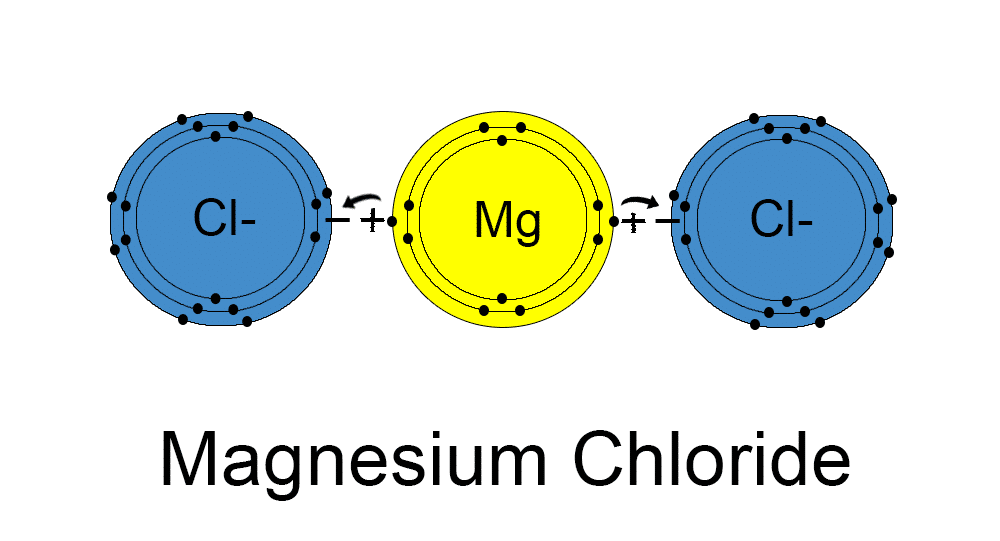
MgCl2 Molecule Ionic Elektra Magnesium

Draw Lewis Structure For Mgcl2

How to Draw the MgCl2 Lewis Dot Structure. YouTube

Explain the formation of the ionic compound magnesium chloride.

A proposed model of saturated MgCl2 solution particles in the rust

Estructura De Lewis Del Mgcl2 Compuesto
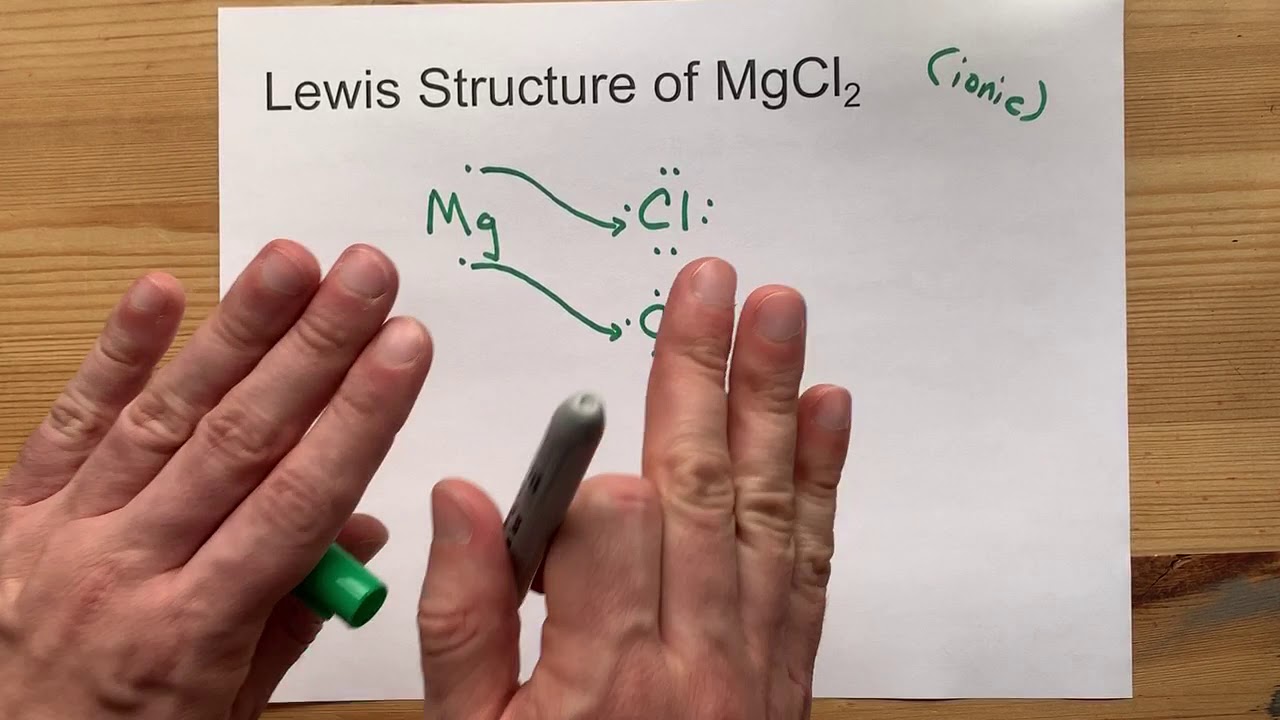
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube
These Valence Electrons Are The Building Blocks Of The Structure Alongside The Individual Atoms.
Molecular Weight/Molar Mass Of Mgcl 2.
Web At The Heart Of Magnesium Chloride’s Usefulness Is Its Unique Set Of Chemical And Physical Properties.
(From Www.superteachertools.net) The Compound Is Held Together By Electrostatic Attractions Between The Positively Charged Magnesium Ion And The Negatively Charged Chloride Ions.
Related Post: