Naming Acids Chart
Naming Acids Chart - The key to success is first realizing that you have an acid. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Oxyacids consist of 3 elements, one of which is oxygen. Hcl(aq) write the formula for each acid. Since all acids contain hydrogen, the name of an acid is based on the anion that goes with it. ) when dissolved in water. Acids without oxygen such as hf, hcl, hbr, hi, hcn and h 2 s: Web the general formula for an acid is h n x, where x is an anion, h is a hydrogen ion, and n is the number of hydrogen ions needed to make the acid neutral. All acids beginning with the. Web the acid name comes from the root name of the anion name. Web naming acids and bases. If you group all the polyatomic ions of chlorine and oxygen from the bonding cheat sheet, a pattern will form: Acids are divided into two groups: Web names and formulas of bases. In simple binary acids, one ion is attached to hydrogen. In simple binary acids, one ion is attached to hydrogen. Acids are divided into two groups: Names for such acids consist of. Web acids have their own nomenclature system. Web naming acids and bases. The key to success is first realizing that you have an acid. Since all acids contain hydrogen, the name of an acid is based on the anion that goes with it. Web the general formula for an acid is h n x, where x is an anion, h is a hydrogen ion, and n is the number of hydrogen ions. Web the acid name comes from the root name of the anion name. Is a compound that contains. Web the name of an acid is derived from the name of its anion. Web names and formulas of bases. Web use this acids and bases chart to find the relative strength of the most common acids and bases. All acids beginning with the. Names of some simple acids; Name of gas name of acid; Binary acids consist of two elements. Web the general formula for an acid is h n x, where x is an anion, h is a hydrogen ion, and n is the number of hydrogen ions needed to make the acid neutral. In simple binary acids, one ion is attached to hydrogen. Oxyacids consist of 3 elements, one of which is oxygen. Web when naming an acid, we can consider the. You can tell because acids begin with h. ) when dissolved in water. All acids beginning with the. In simple binary acids, one ion is attached to hydrogen. Oxyacids consist of 3 elements, one of which is oxygen. If you group all the polyatomic ions of chlorine and oxygen from the bonding cheat sheet, a pattern will form: Web names and formulas of bases. ) when dissolved in water. Web the good news is that naming bases is going to be pretty simple for you. Web the general formula for an acid is h n x, where x is an anion, h is a hydrogen ion, and n is the number of hydrogen ions needed to make the acid neutral. The key to success. Is a compound that contains. Web when naming an acid, we can consider the. Web the good news is that naming bases is going to be pretty simple for you. Acids without oxygen such as hf, hcl, hbr, hi, hcn and h 2 s: Naming acids is divided in two categories: Name of gas name of acid; ) when dissolved in water. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Is a compound that contains. In simple binary acids, one ion is attached to hydrogen. Name of gas name of acid; All acids beginning with the. The key to success is first realizing that you have an acid. Web acids have their own nomenclature system. Binary acids consist of two elements. Naming acids is divided in two categories: Web the acid name comes from the root name of the anion name. Namely, since most of them are hydroxides (naoh, koh, etc.), you just name them like. Making connections direct students to lesson 9.2 and review the guidelines for naming and writing ionic compounds. Acids without oxygen such as hf, hcl, hbr, hi, hcn and h 2 s: Web be the first to ask a question about this topic. Since all acids contain hydrogen, the name of an acid is based on the anion that goes with it. These are the correlation patterns for the names of monoatomic and polyatomic (oxoanions) ions and their acids:. Web naming acids and bases author: You can tell because acids begin with h. Acids are divided into two groups:
Nomenclature of Acids Pathways to Chemistry
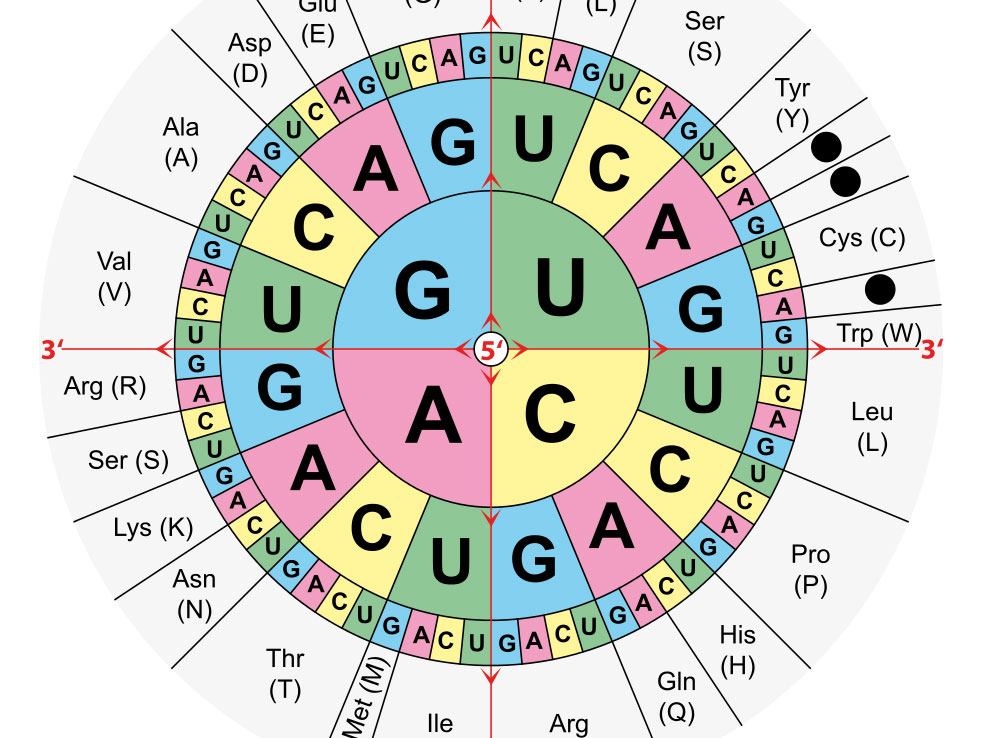
Amino Acids table Wikimedia Commons Biosciences Area

Compound Interest A Brief Guide to the Twenty Common Amino Acids

Classification of Acids on Basis of source, Concentration Teachoo

Everything You Need to Know About Amino Acids IV Boost UK
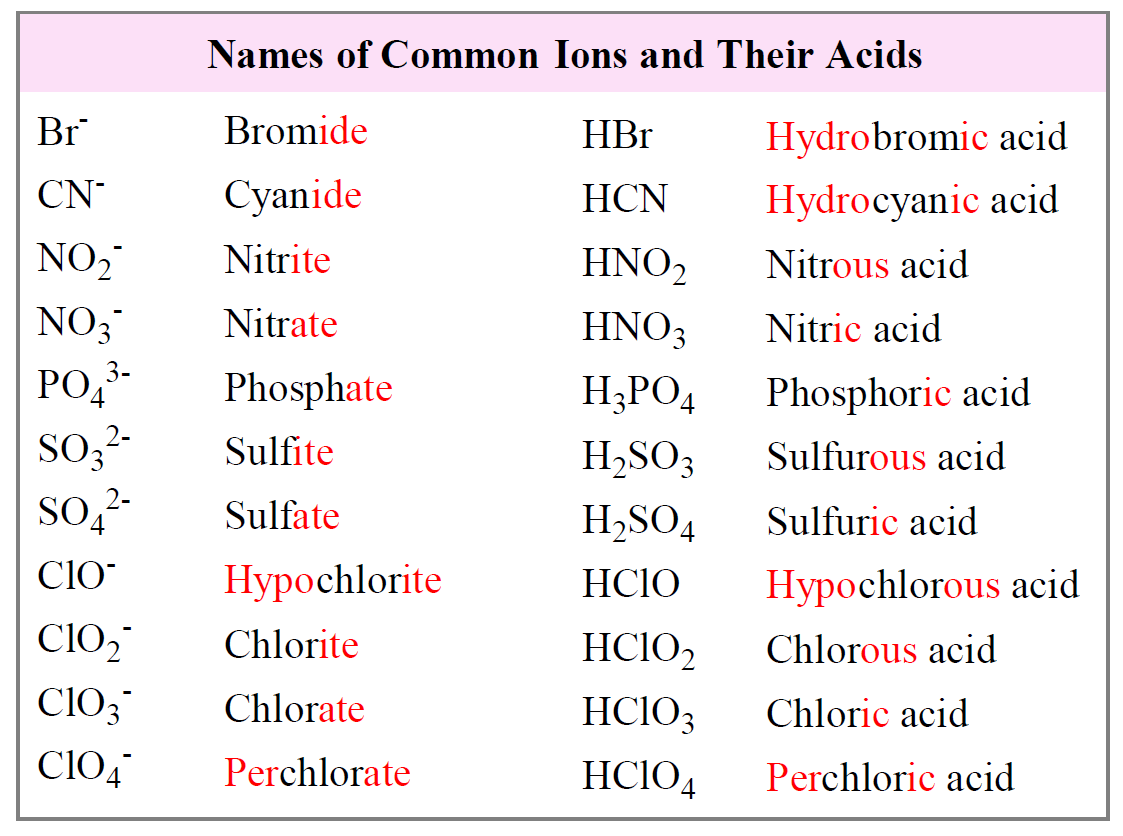
Naming Acids and Bases Chemistry Steps
Chemical Nomenclature and Chemical Formulas Owlcation
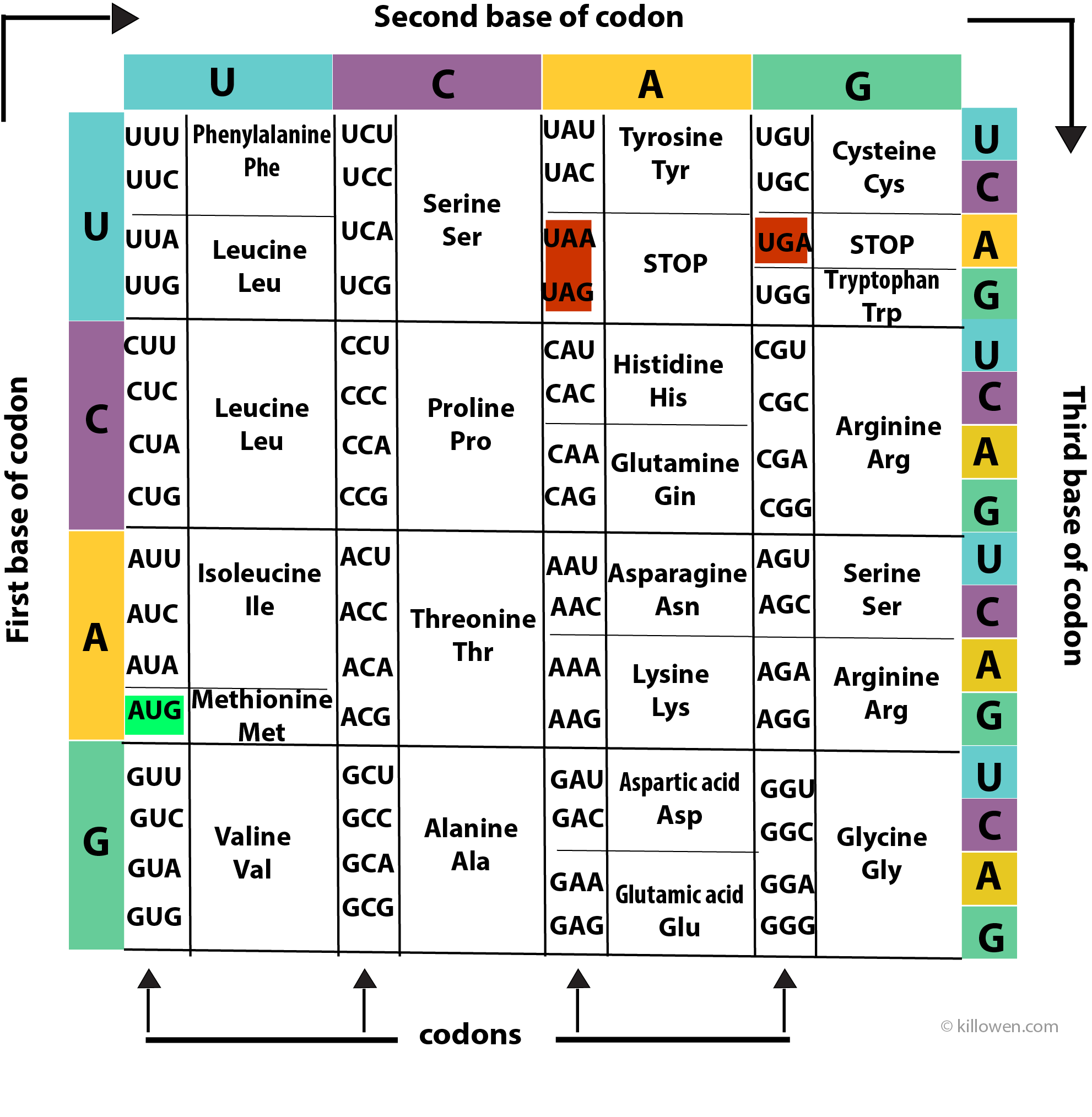
Amino Acids Coding Structure
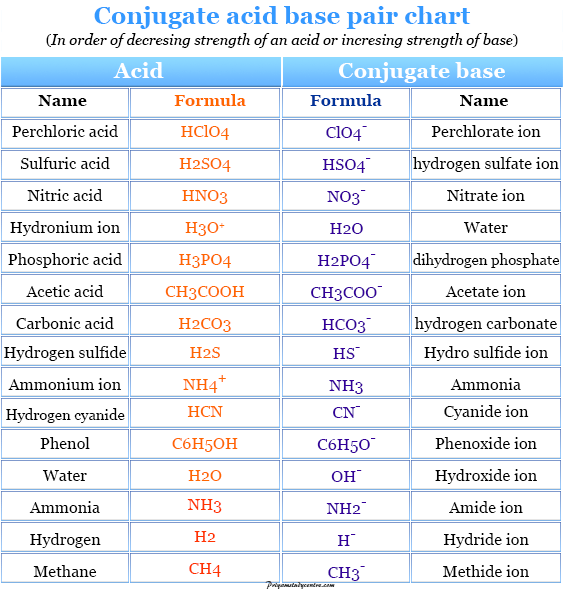
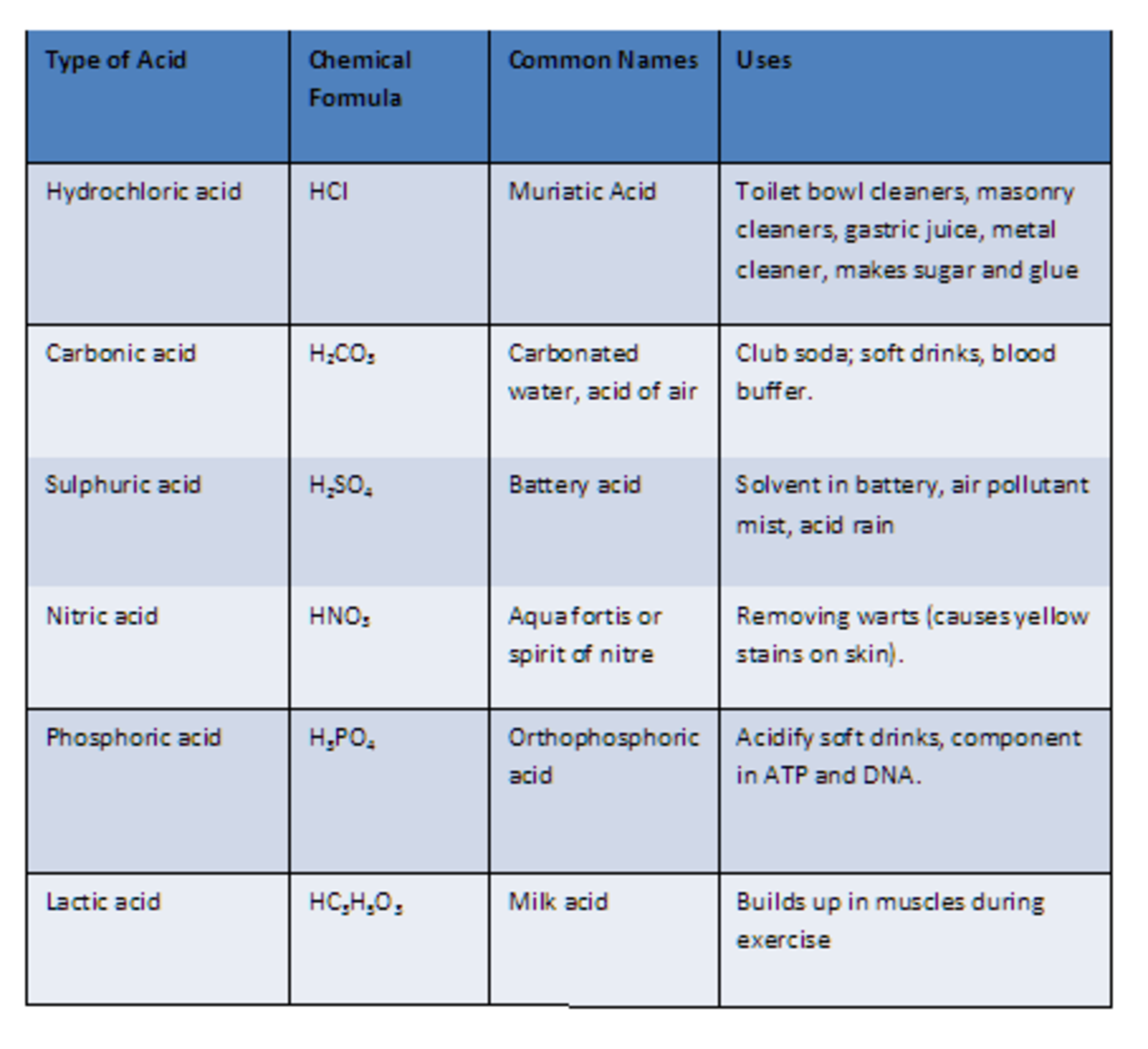
Acid And Base Chart
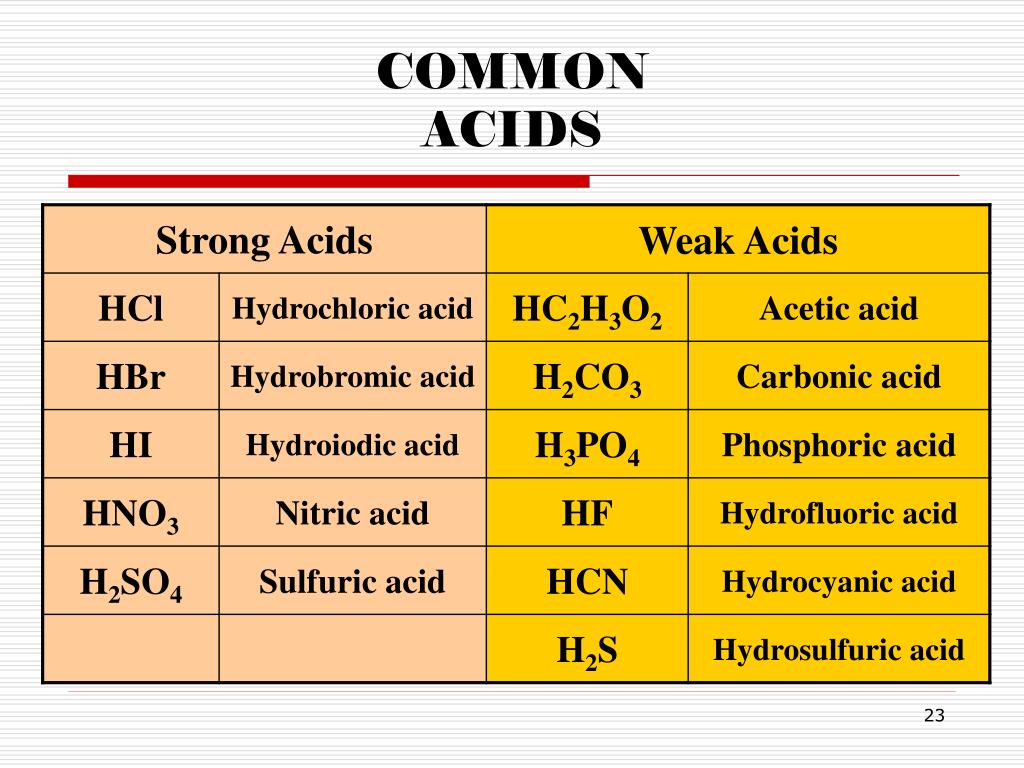
Chemistry Naming Acids Rules And Practice vrogue.co
If You Group All The Polyatomic Ions Of Chlorine And Oxygen From The Bonding Cheat Sheet, A Pattern Will Form:
Hcl(Aq) Write The Formula For Each Acid.
) When Dissolved In Water.
Oxyacids Consist Of 3 Elements, One Of Which Is Oxygen.
Related Post: