Multihance Dose Chart
Multihance Dose Chart - • for mri of the cns in pediatric. Gadoxetic acid (eovist) 0.25 m: Multihance generic name & formulations. Since the drug is substantially. Last updated on sep 12, 2023. Dose at uw is twice package insert. Web multihance is cleared from the body mainly by glomerular filtration (85% to 95%) and to a minor degree (0.6% to 4.0%) by hepatobiliary excretion. Nausea, headache, feeling hot, or burning at the injection site. Web standard dose (mmol/kg) package insert; Gadobenate dimeglumine (multihance) 0.5 m: — therapeutic categories — imaging agents. Web standard dose (mmol/kg) package insert; Web therefore, the recommended dose of multihance in adult patients and children is 0.05 mmol/kg body weight (0.1 ml/kg of the 0.5 m solution). Web 6 multihance ® (gadobenate dimeglumine) injection, 529 mg/ml full prescribing information and patient medication guide. Web the recommended dose of multihance is 0.2. Web or 0.1 mmol/kg of multihance. Web medically reviewed by drugs.com. Web the recommended dose of multihance is 0.2 ml/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection. Package insert dose of eovist is 0.025mmol/kg. Dose at uw is twice package insert. Web medically reviewed by drugs.com. Web the recommended dose of multihance® in adult patients is 0.05 mmol/kg body weight. Since the drug is substantially. These are not all the possible side effects of multihance. Web multihance is cleared from the body mainly by glomerular filtration (85% to 95%) and to a minor degree (0.6% to 4.0%) by hepatobiliary excretion. Web therefore, the recommended dose of multihance in adult patients and children is 0.05 mmol/kg body weight (0.1 ml/kg of the 0.5 m solution). 1 agents currently on formulary at uw. Web multihance is cleared from the body mainly by glomerular filtration (85% to 95%) and to a minor degree (0.6% to 4.0%) by hepatobiliary excretion. Web gadolinium based contrast. Dose at uw is twice package insert. — therapeutic categories — imaging agents. Since the drug is substantially. It is used in combination with magnetic resonance imaging (mri) or. Multihance is used with a magnetic resonance imaging (mri) scanner to see. Nausea, headache, feeling hot, or burning at the injection site. Web the following contrast agent/strength will be utilized: This corresponds to 0.1 ml/kg of the 0.5 m solution. • for mri of the cns in pediatric patients below 2 years of. Gadoxetic acid (eovist) 0.25 m: Web medically reviewed by drugs.com. Multihance is a contrast agent that has magnetic properties. Web the recommended dose of multihance® in adult patients is 0.05 mmol/kg body weight. Since the drug is substantially. Web for mra examination, the recommended dose is 0.2 ml/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection followed by at least 20 ml saline. • for mri of the cns in pediatric patients below 2 years of. • for mri of the cns in pediatric. Web the following contrast agent/strength will be utilized: This corresponds to 0.1 ml/kg of the 0.5 m solution. Of these 150 patients, 74 received 0.05 mmol/kg of multihance as the first dose and 76 received 0.1 mmol/kg of multihance. Web the recommended dose of multihance is 0.2 ml/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection. Gadobenate dimeglumine (multihance) 0.5 m: Web the following contrast agent/strength will be utilized: 1 agents currently on formulary at uw. Of these 150 patients, 74 received 0.05 mmol/kg of multihance as the first dose and 76 received 0.1 mmol/kg of multihance as. Web the most common side effects of multihance include: Dose at uw is twice package insert. Web • the recommended dose of multihance is 0.2 ml/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection. Nausea, headache, feeling hot, or burning at the injection site. The lowest dose that provides. Multihance is a contrast agent that has magnetic properties. • for mri of the cns in pediatric patients below 2 years of. Package insert dose of eovist is 0.025mmol/kg. Web or 0.1 mmol/kg of multihance. Web the recommended dose of multihance® in adult patients is 0.05 mmol/kg body weight. Of these 150 patients, 74 received 0.05 mmol/kg of multihance as the first dose and 76 received 0.1 mmol/kg of multihance as the. — therapeutic categories — imaging agents. Nausea, headache, feeling hot, or burning at the injection site. Web 6 multihance ® (gadobenate dimeglumine) injection, 529 mg/ml full prescribing information and patient medication guide. Web therefore, the recommended dose of multihance in adult patients and children is 0.05 mmol/kg body weight (0.1 ml/kg of the 0.5 m solution). Web the following contrast agent/strength will be utilized: Web multihance is cleared from the body mainly by glomerular filtration (85% to 95%) and to a minor degree (0.6% to 4.0%) by hepatobiliary excretion. Web gadolinium based contrast dosing charts: Since the drug is substantially. Web the recommended dose of multihance is 0.2 ml/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection. The lowest dose that provides.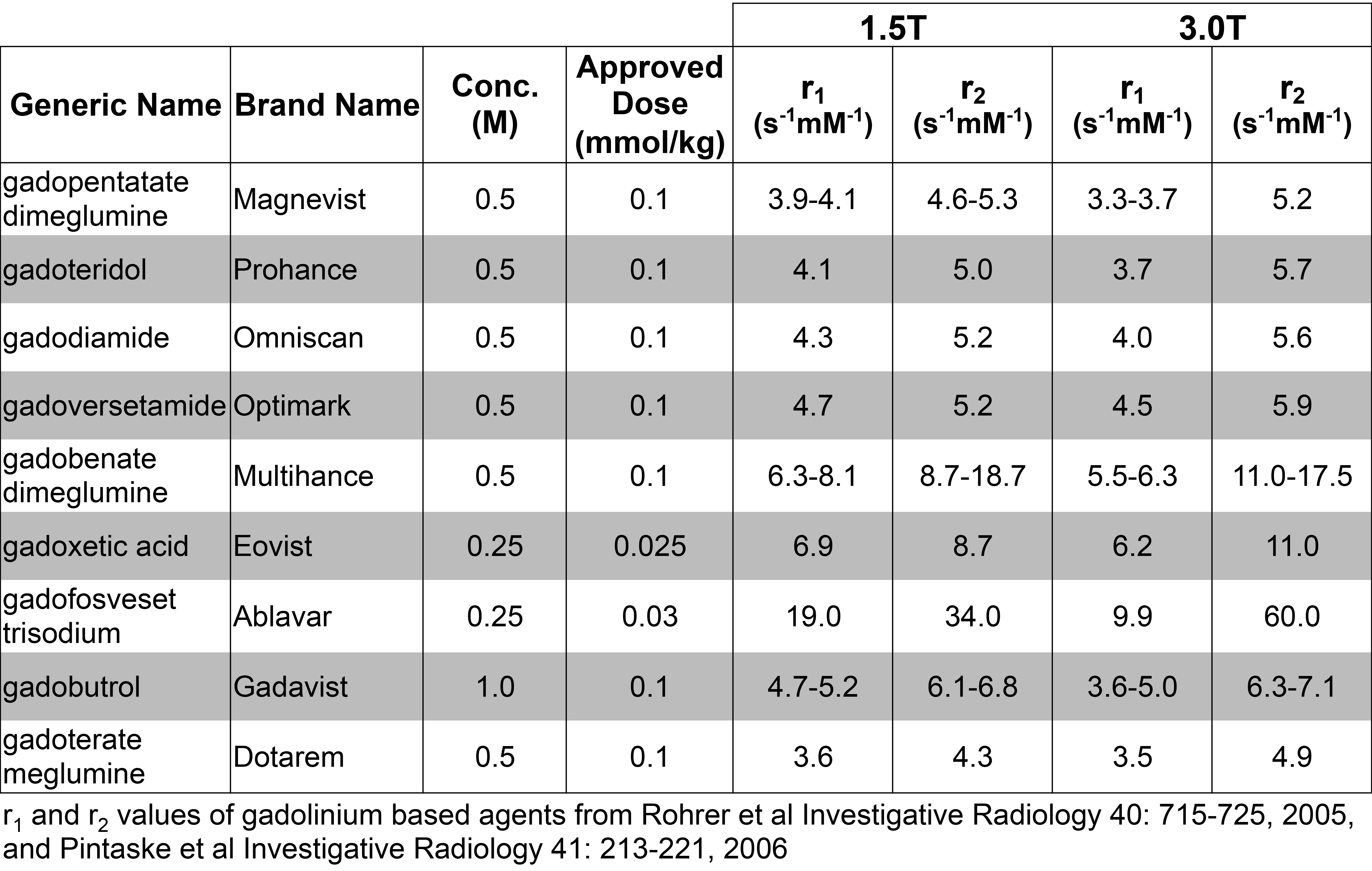
Table 1 List of approvedgadoliniumbased contrast agents including
Gadobenate Dimeglumine wikidoc
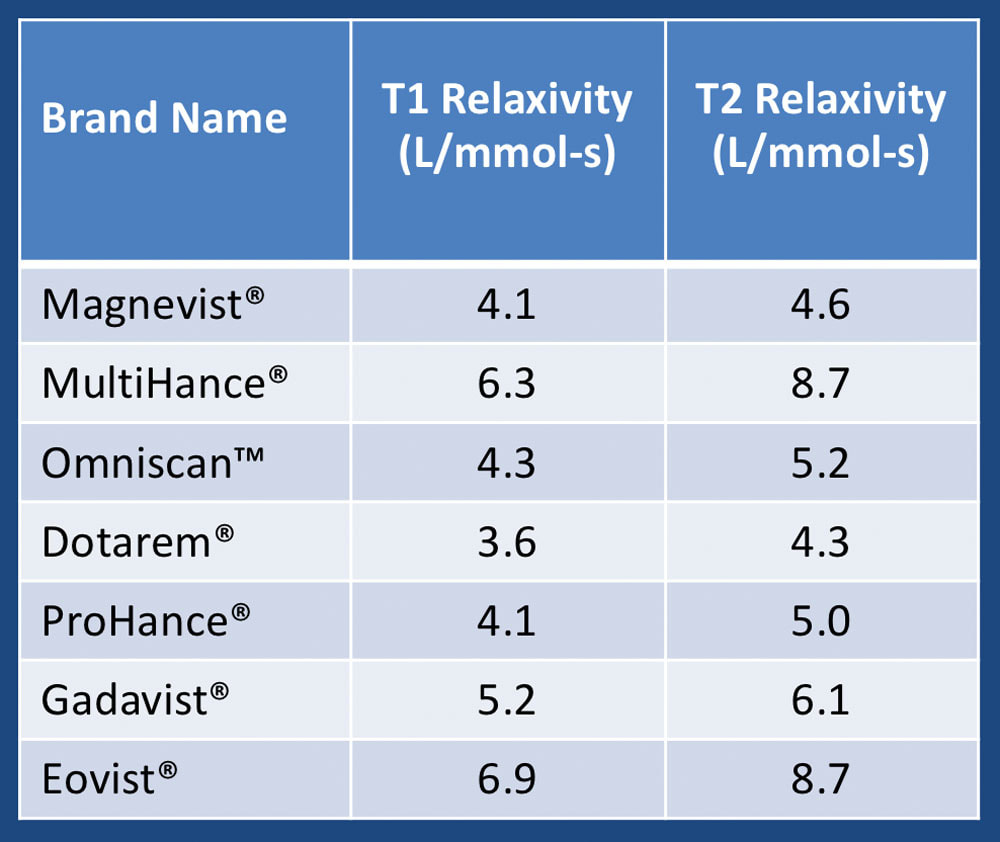
Relaxivity Questions and Answers in MRI
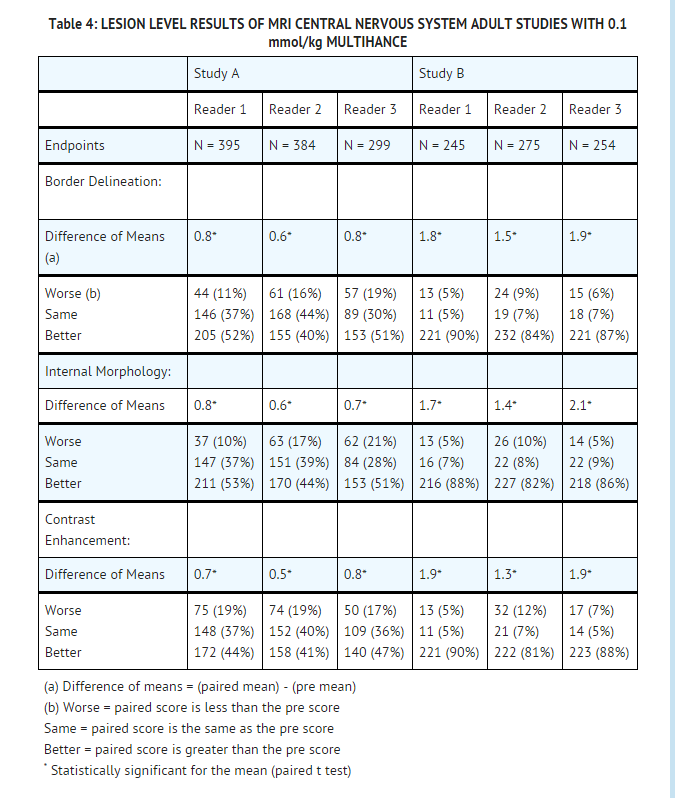
Multihance Dose Chart
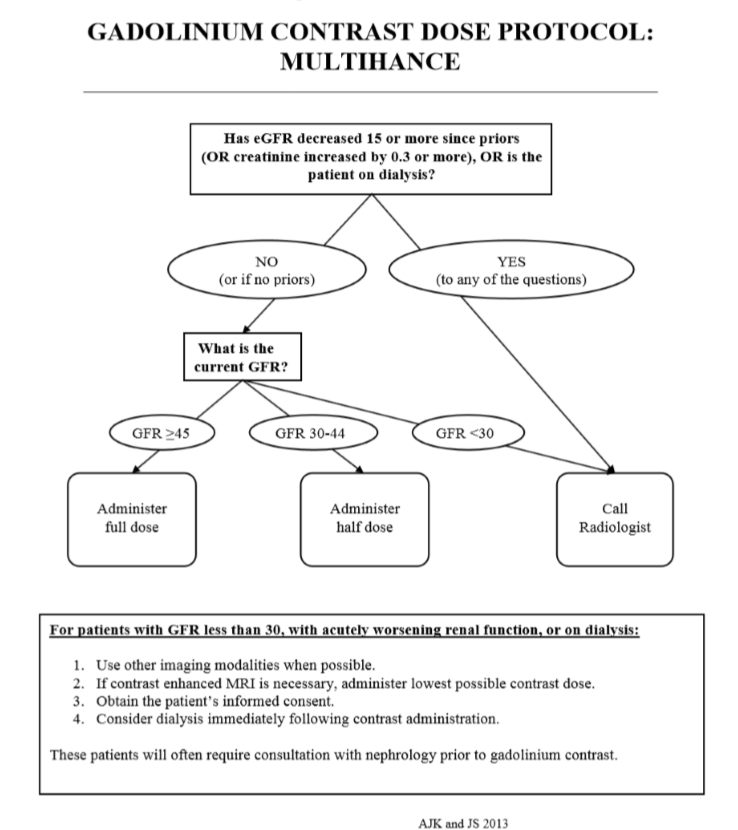
Multihance Dose Chart
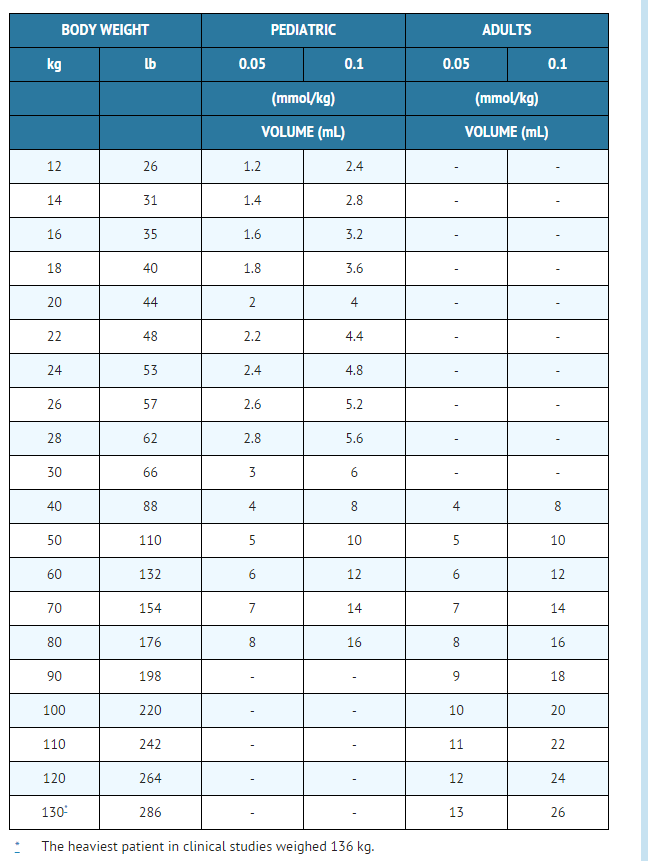
Gadodiamide wikidoc
MultiHance Package Insert

Multihance Dose Chart

Multihance Dose Chart

Multihance Dose Chart
These Are Not All The Possible Side Effects Of Multihance.
For Mri Of The Cns In Pediatric.
• For Mri Of The Cns In Pediatric.
Web • The Recommended Dose Of Multihance Is 0.2 Ml/Kg (0.1 Mmol/Kg) Administered As A Rapid Bolus Intravenous Injection.
Related Post: