Mole Conversions Chart
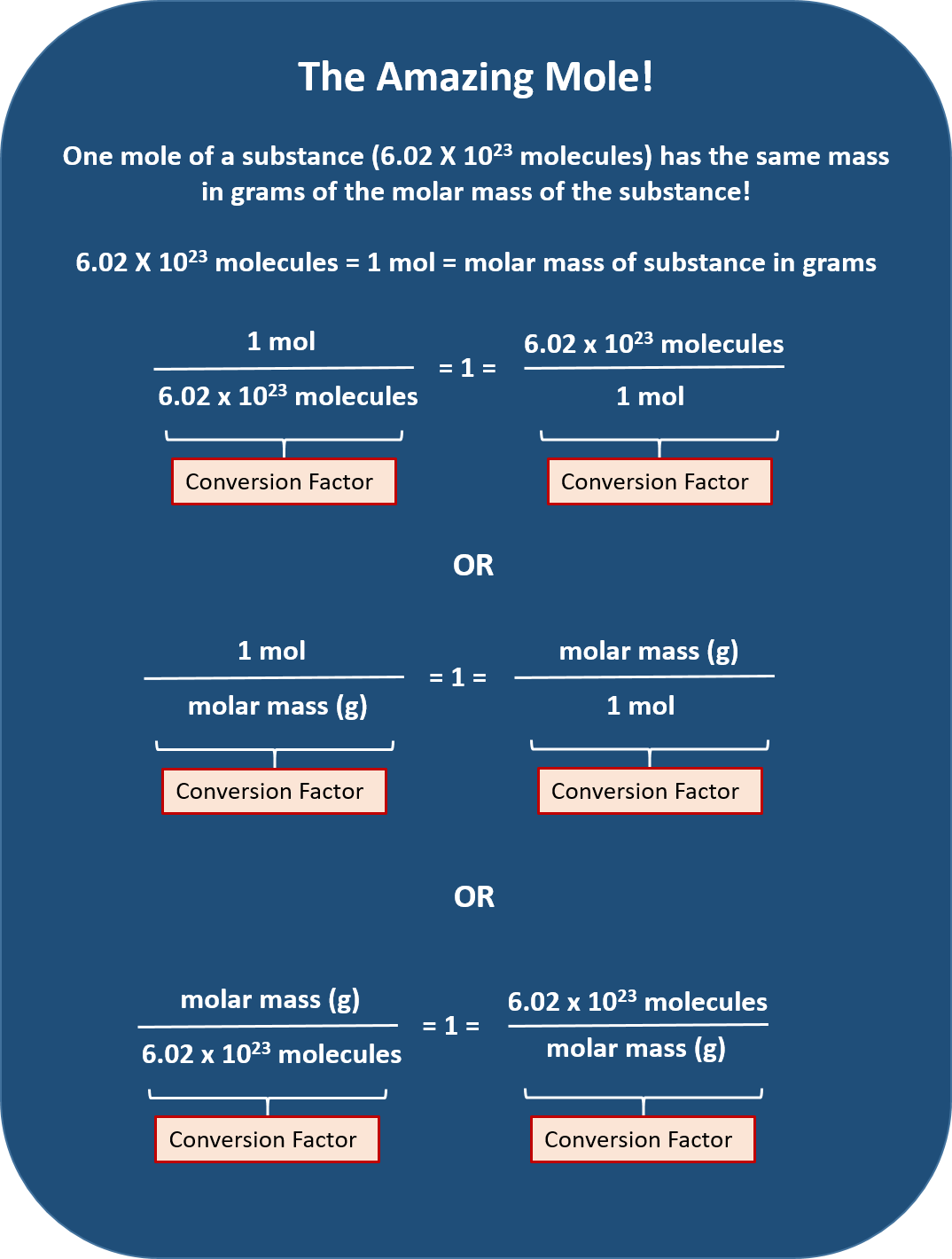
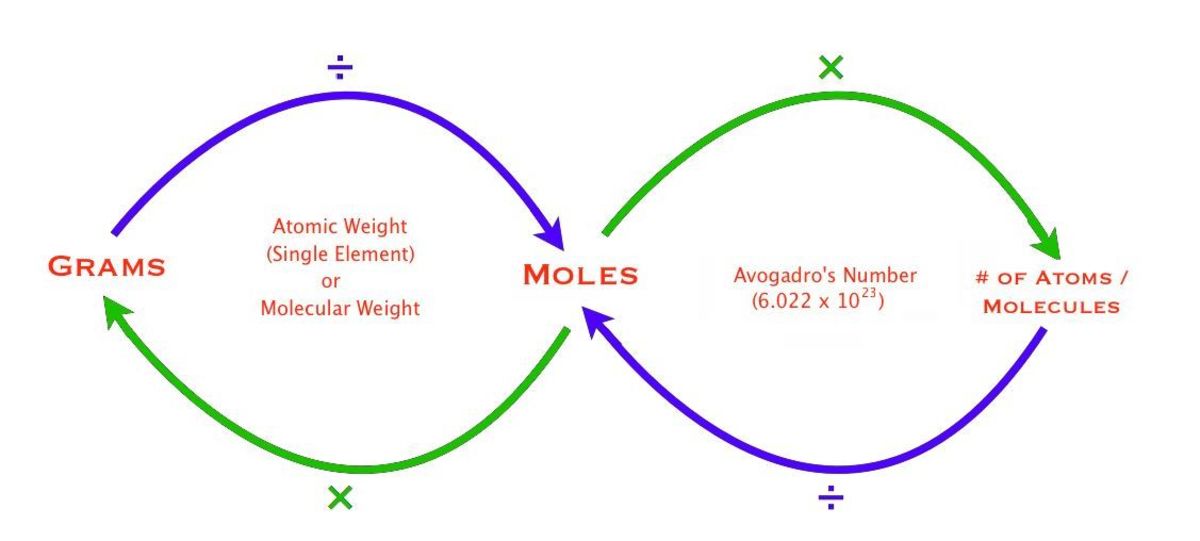
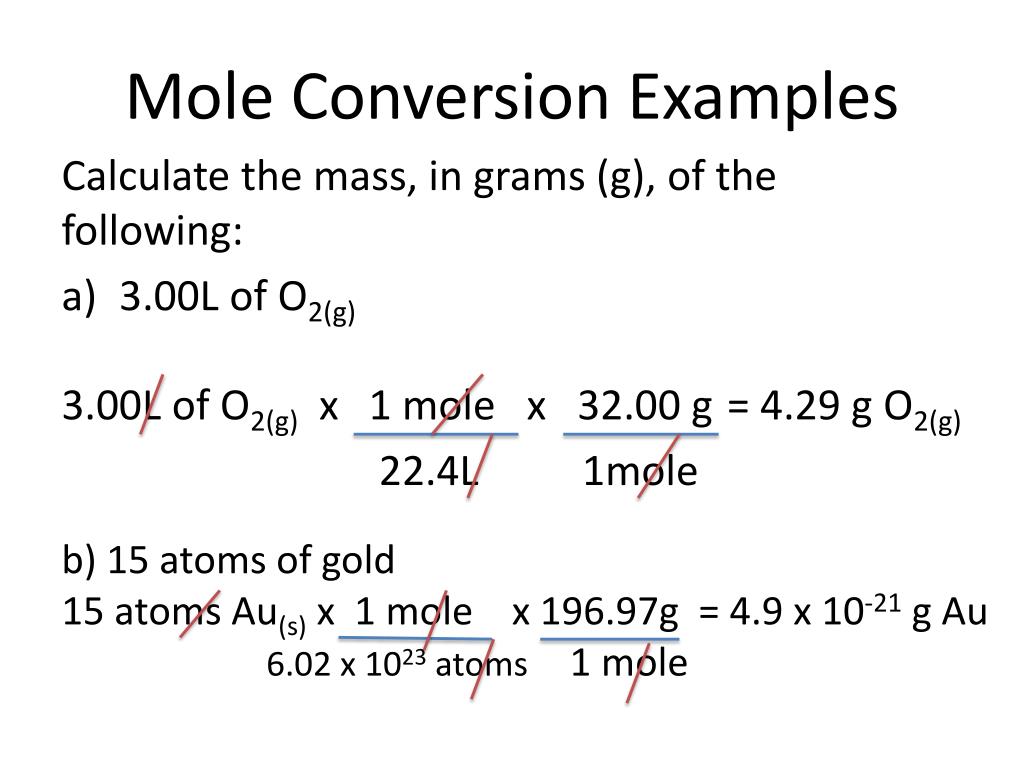
Mole Conversions Chart - The previous section described the molar interpretation of a balanced chemical equation. How to use this mole bridge: Web how many moles of salt are in 13.8 g of sodium chloride? Web whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole conversion: Web moles are a type of unit conversion used in chemistry to measure the amount of substances. Web you can calculate the molar mass of a molecule by summing the molar masses of the composing atoms with the help of the periodic table. Click 'calculate' to get the results instantly. If you have 22.414 grams of cl 2, how many moles of molecular chlorine do you have? Divide your initial mass by the molar mass of the compound as determined by the periodic table. Express the answer using 3 significant figures. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and back. Web you can calculate the molar mass of a molecule by summing the molar masses of the composing atoms with the help of the periodic table. Web figure \(\pageindex{1}\) is a chart for determining what conversion factor is needed,. At first, use the mole map to set up and solve problems. Web your guide to mole conversions. Click 'calculate' to get the results instantly. Mass concept builder includes three scaffolded difficulty levels designed to provide learners comfort with the mathematical relationship between the number of particles of a compound, the number of. Web how many moles of salt are. Example 3 in section 6.2 “atomic and molar masses” stated that the mass of 2 mol of u is twice the molar mass of uranium. Using our unit conversion techniques, we can use the mole label to convert back and forth between the number of particles and moles. Web perform conversions between mass and moles of a substance. The previous. Divide your particle value by avogadro's number, 6.02×10 23. The great divide side, you will divide. Web determine the mass of 0.752 mol of h 2 gas. Example 3 in section 6.2 “atomic and molar masses” stated that the mass of 2 mol of u is twice the molar mass of uranium. Web in this video you'll learn to use. Click 'calculate' to get the results instantly. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Web in this video you'll learn to use the mole map to visually build a plan to convert from moles. N 2 ( g) + 3 h 2 ( g) → 2 nh 3 ( g) tells us that 1 mol n 2 reacts with 3 mol h 2 to yield 2 mol nh 3. By converting from moles to grams, liters, and other units, chemists can calculate the mass or volume of a given substance. Such a straightforward exercise. Remember to use parentheses on your calculator! Enter the amount you wish to convert (in moles or grams). The study of the numerical relationships between the reactants and the products in balanced chemical reactions is called stoichiometry. We have provided many ways for you to learn about this topic. Using our unit conversion techniques, we can use the mole label. If you have 22.414 grams of cl 2, how many moles of molecular chlorine do you have? Convert quantities between mass units and mole units. At first, use the mole map to set up and solve problems. Use a balanced chemical equation to determine molar relationships between substances. Web we can use these ratios to determine what amount of a. We have provided many ways for you to learn about this topic. Web determine the mass of 0.752 mol of h 2 gas. Always plan problems so you can cancel units. On the rabittville, side you will be multiplying (rabbits multiply). Web whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use. Enter the amount you wish to convert (in moles or grams). The great divide side, you will divide. From mass (grams) to moles: Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Web the physics classroom » calculator pad » mole conversions » equation overview. Enter the amount you wish to convert (in moles or grams). Divide your particle value by avogadro's number, 6.02×10 23. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. Web conversions between moles and number of particles. Mass concept builder is quite simple. Multiply your mole value by the molar volume constant, 22.4l. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and back. You will be presented with a table of numerical values associated with molar mass, number of particles, number of moles, and the mass in grams for a stated substance. How many moles of molecular hydrogen are present in 6.022 grams of h 2? Explore the features that make our mole calculator superior. Web perform conversions between mass and moles of a substance. Web use a balanced chemical equation to determine molar relationships between substances. Web the physics classroom » calculator pad » mole conversions » equation overview. Web converting from moles to volume (liters): Web in this video you'll learn to use the mole map to visually build a plan to convert from moles to grams with six typical mole to grams conversion problems. The great divide side, you will divide.
Mole Conversion Cheat Sheet

Mole Conversion chart Diagram Quizlet
Chapter 6 Quantities in Chemical Reactions Chemistry

Mole Conversions and Calculation SSC Chemistry
Grams To Moles Conversion Chart

Chemistry Mole Conversion Chart Images & Pictures Becuo Chemistry
Chemistry Mysteries Mole Conversions

Molar Mass Conversion Chart
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free
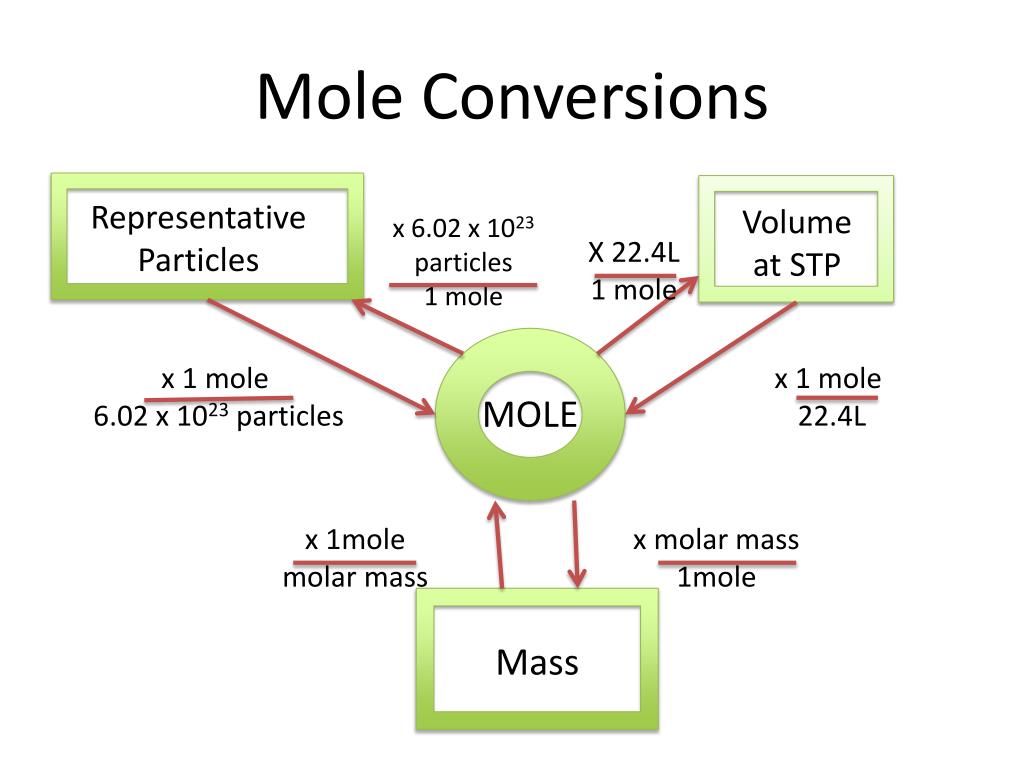
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free
Such A Straightforward Exercise Does.
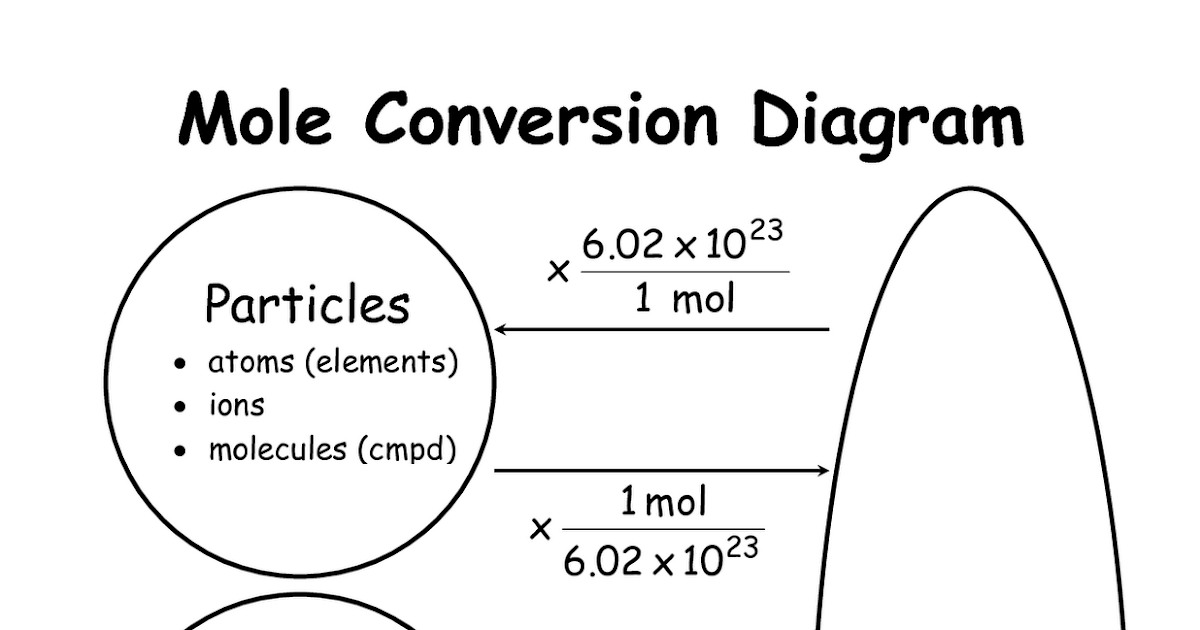
Converting From Particles (Atoms, Molecules, Or Formula Units) To Moles:
You Are Only Allowed To Go From Left To Right.
By Converting From Moles To Grams, Liters, And Other Units, Chemists Can Calculate The Mass Or Volume Of A Given Substance.
Related Post: