Matter Chart Chemistry
Matter Chart Chemistry - Atoms or molecules are in close proximity, but individual atoms and molecules can pass across one another. We can see that they take up space, and their weight tells us that they have mass. Density, colour, hardness, melting and boiling points, and electrical conductivity are all examples of physical properties. Complex molecules can also form various mesophases such as liquid crystals, which are intermediate between the liquid and solid phases. Want to join the conversation? We can see that they take up space, and their weight tells us that they have mass. Web in classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Web in common temperatures and pressures, atoms form the three classical states of matter: Web a matter flowchart is a diagram usually used widely in chemistry to classify matter. Web matter can be identified by its characteristic inertial and gravitational mass and the space that it occupies. Atoms or molecules are in close proximity, but individual atoms and molecules can pass across one another. Identify a sample of matter as an element, a compound, or a mixture (homogeneous/heterogeneous). Under exceptional conditions, other states of matter also exist. There is. If gases did not take up space, a balloon would not inflate (increase its volume) when filled with gas. Web matter is defined as anything that occupies space and has mass, and it is all around us. Web produced by nina feldman , clare toeniskoetter , rob szypko and diana nguyen. Web in classical physics and general chemistry, matter is. Web matter can be classified according to physical and chemical properties. Solids and liquids are more obviously matter: At a minimum, matter requires at least one subatomic particle, although most matter consists of atoms. Atoms or molecules are in close proximity, but individual atoms and molecules can pass across one another. The word matter is sometimes used to refer to. Solids and liquids are more obviously matter: Under exceptional conditions, other states of matter also exist. If gases did not take up space, a balloon would not inflate (increase its volume) when filled with gas. Connect daily observations to molecular interactions using electronegativity, bond polarity, and intermolecular forces. Matter is anything that has mass and takes up space. A chemical substance is composed of one type of atom or molecule. Atoms or molecules are in close contact, often in a highly organized arrangement. Web matter can be classified into two broad categories: Visit byju’s to learn more about it. Density, colour, hardness, melting and boiling points, and electrical conductivity are all examples of physical properties. A solid has a definite shape and volume. Solid matter is composed of tightly packed particles. Matter is anything that occupies space and has mass. Matter can be broken down into two categories: Label all atoms within an organic or inorganic compound. Web matter is defined as anything that occupies space and has mass, and it is all around us. Visit byju’s to learn more about it. Distinguish between physical and chemical changes. Mixtures are physically combined structures that can be separated into their original components. Distinguish between mass and weight. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Web matter is defined as anything that occupies space and has mass, and it is all around us. Matter is typically commonly found in three different states: The three most common states or phases of matter are solid, liquid,. Identify a sample of matter as an element, a compound, or a mixture (homogeneous/heterogeneous). Web in common temperatures and pressures, atoms form the three classical states of matter: Pure substances are further broken down into elements and compounds. The three most common states or phases of matter are solid, liquid, and gas. Web matter is defined as anything that occupies. Want to join the conversation? Web a matter flowchart is a diagram usually used widely in chemistry to classify matter. Web in science, matter is the term for any type of material. Why doesn't it change temperature? The matter will be categorized into two types: Under exceptional conditions, other states of matter also exist. A solid has a definite shape and volume. Original music by marion lozano , elisheba ittoop and sophia lanman. Want to join the conversation? Solid matter is composed of tightly packed particles. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Distinguish between physical and chemical changes. Web in common temperatures and pressures, atoms form the three classical states of matter: Web describe the different physical states of matter; Apply the law of conservation of matter. A physical property is an attribute of matter that is independent of its chemical composition. Where does the periodic table come from? Web use physical and chemical properties, including phase, to describe matter. The three most common states or phases of matter are solid, liquid, and gas. Liquid matter is made of more loosely packed particles. Distinguish between mass and weight.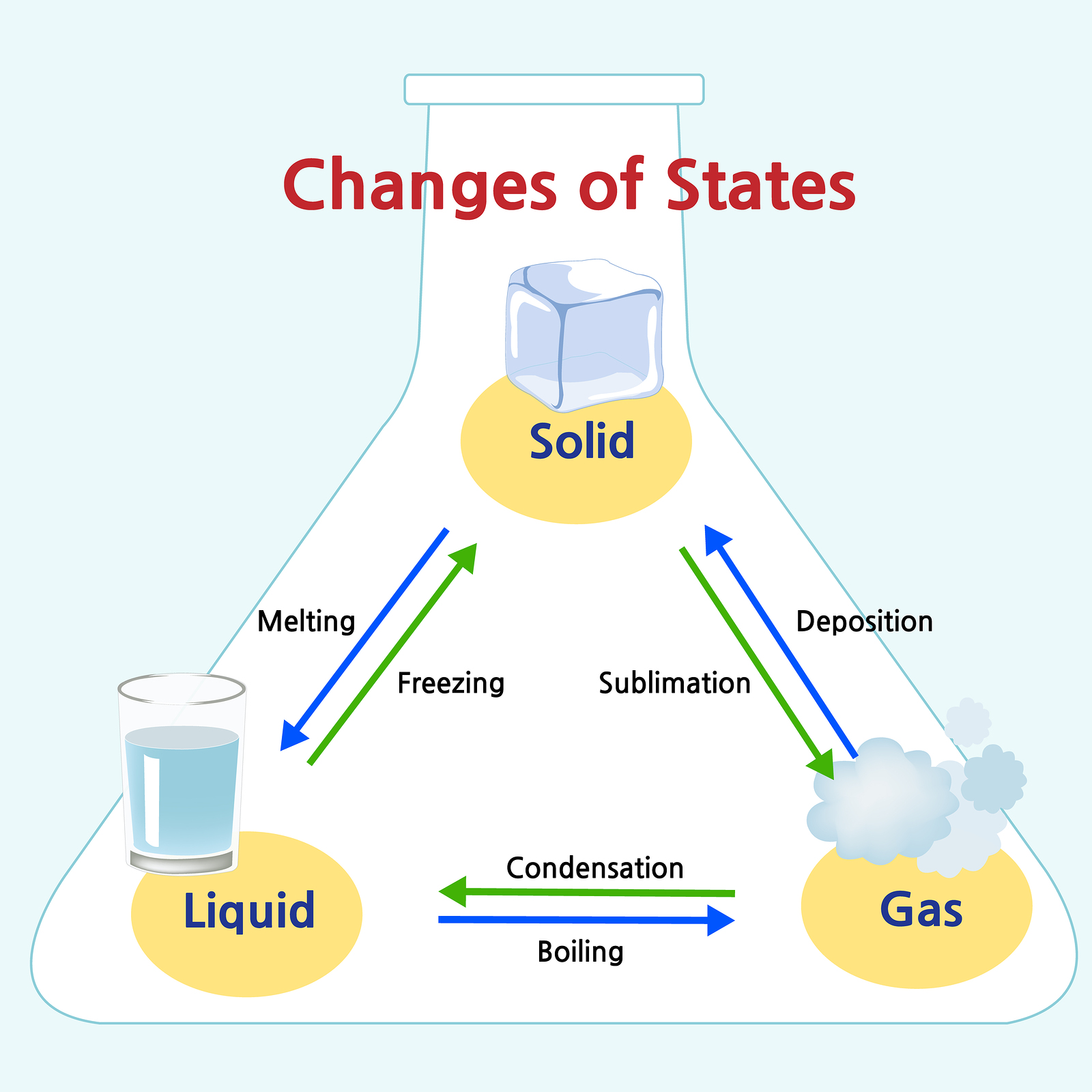
What are states of matter? TheSchoolRun
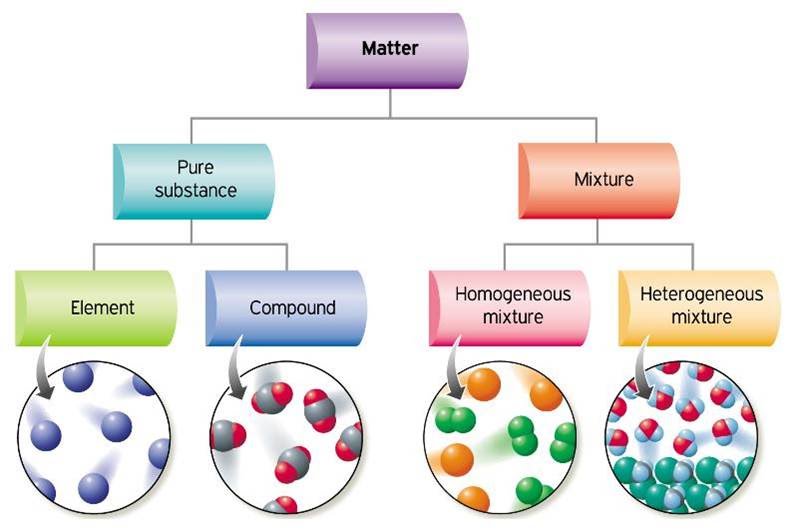
Classification of matter Chemistry 10

Grade 9 Chemistry The Classification of Matter K12Science FORBEST
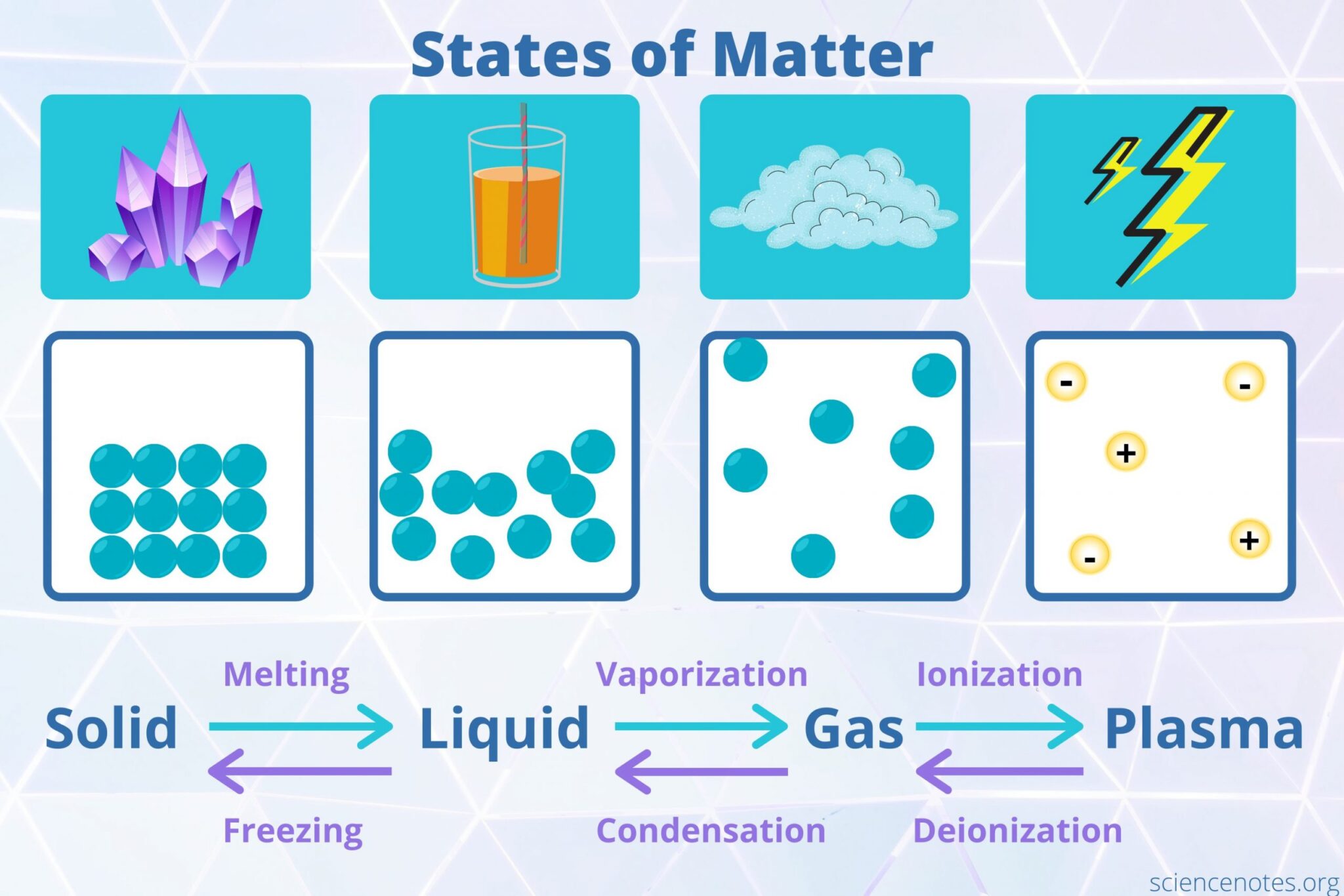
States of Matter Teach Kids Chemistry
/GettyImages-947148218-1742a84786ae46b0b229da848348fb0f.jpg)
State of Matter Definition Chemistry Glossary
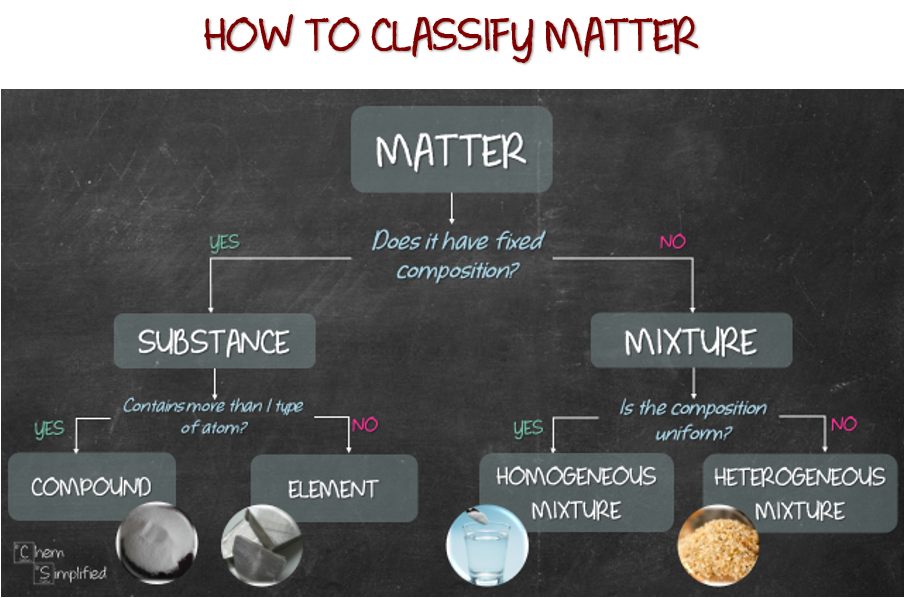
2.1 Classifications of Matter Chemistry LibreTexts
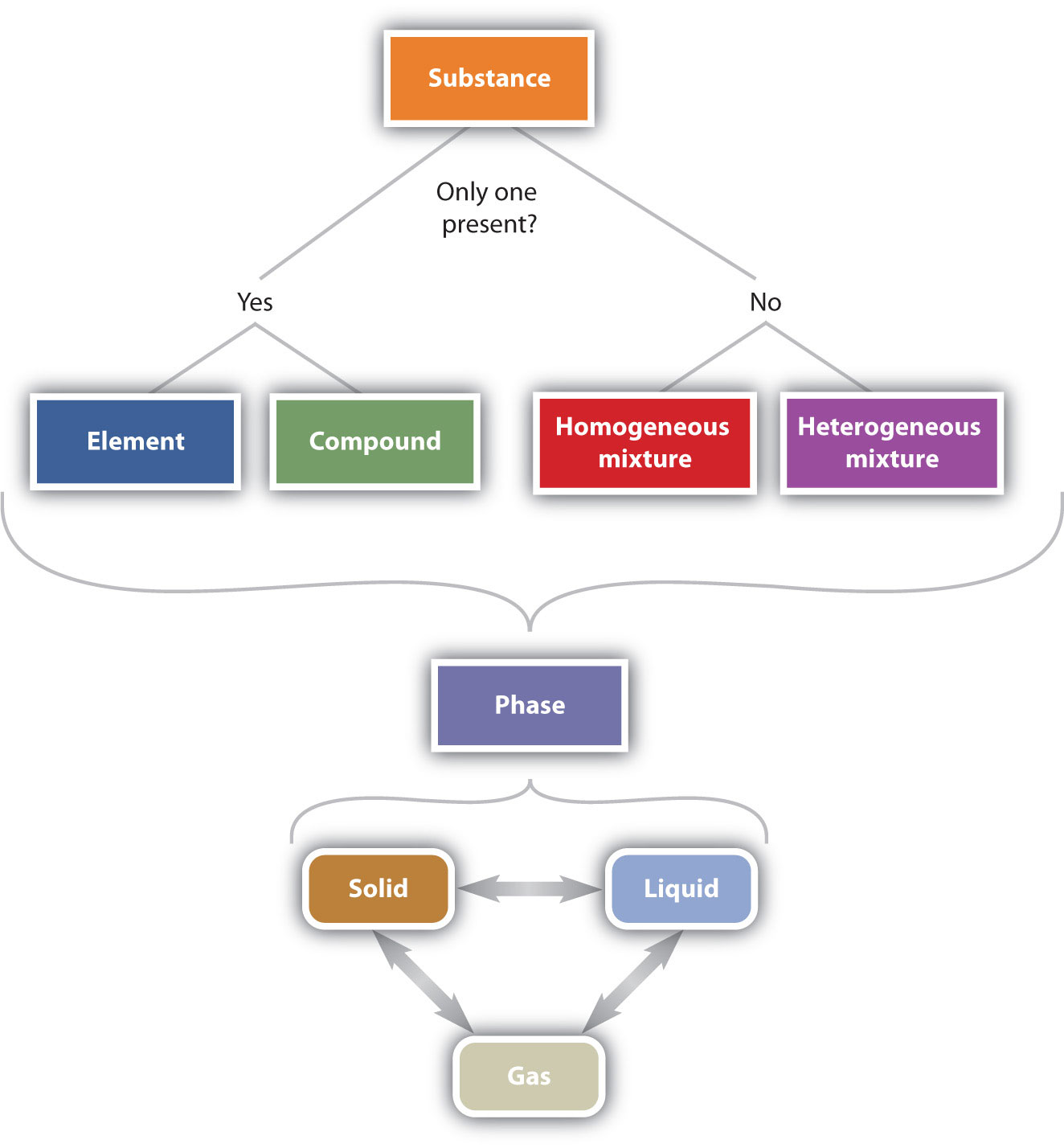
1.2 The Classification of Matter Chemistry LibreTexts
:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png)
List of Phase Changes Between States of Matter
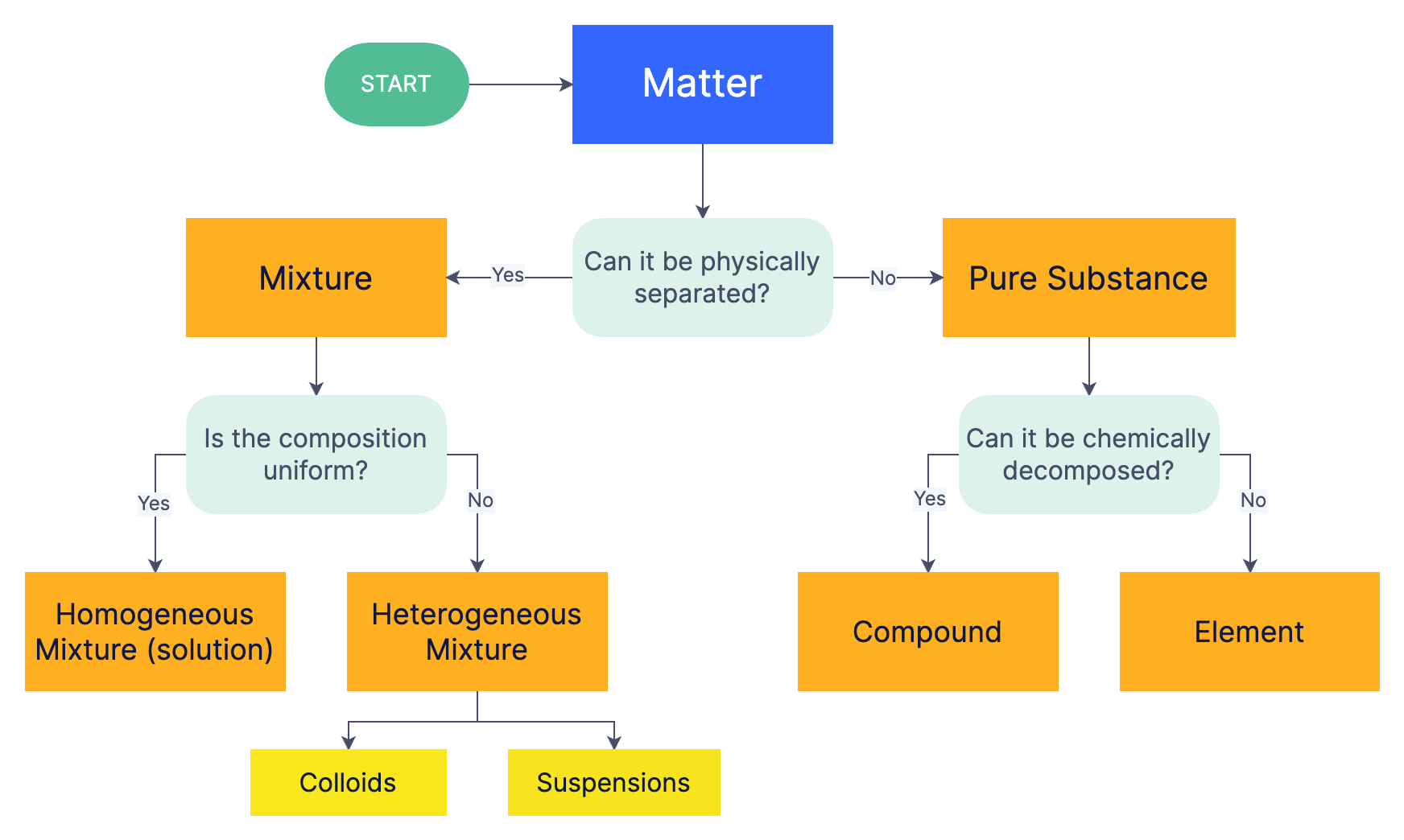
Matter Flowchart Visual Guide to Classify Matter
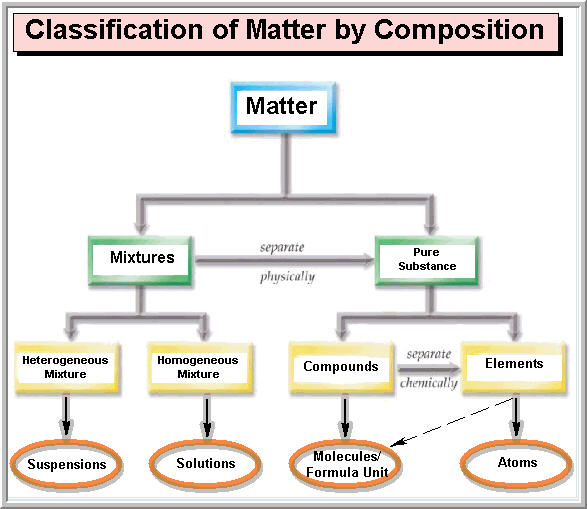
Matter Classification Of Matter Flowchart Classificat vrogue.co
Mixtures Are Physically Combined Structures That Can Be Separated Into Their Original Components.
Solids And Liquids Are More Obviously Matter:
If Gases Did Not Take Up Space, A Balloon Would Stay Collapsed Rather Than Inflate When Filled With Gas.
I Mean Where/What Does The Energy Go/Do?
Related Post: