Hybridization Geometry Chart
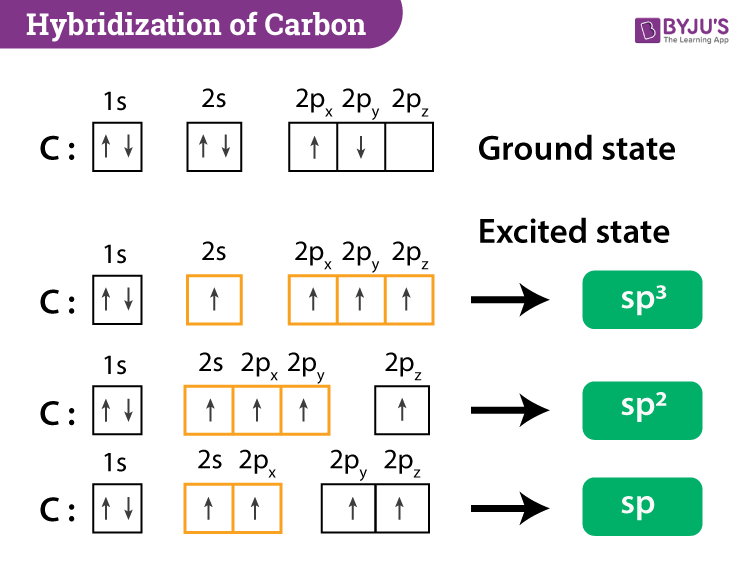
Hybridization Geometry Chart - Get the free hybridization widget for your website, blog, wordpress, blogger, or igoogle. Web hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridisation and geometry of molecules play a vital role in their reactivity. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. Want to join the conversation? Reactions involve making and breaking of bonds! Covalent bonds are formed by: Web hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure 3). Web the hybridization of an atom is determined based on the number of regions of electron density that surround it. Add these two numbers together. Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. Hybridisation and geometry of molecules play a vital role in their reactivity. Web sp³, sp² and sp hybridization, or the mixing of s and p orbitals which allows us to create sigma and. Web learn the definition of orbital hybridization and the characteristics and geometries of sp, sp2, sp3, sp3d1, and sp3d2 hybridization. The geometrical arrangements characteristic of the various sets of hybrid orbitals are shown in figure 8.21. Web molecular geometry van koppen/offen procedure: Web hybridization increases the overlap of bonding orbitals and explains the molecular geometries of many species whose geometry. Find more chemistry widgets in. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). In this video, we use both of these methods to determine the hybridizations. So, before we start with organic chemistry, let's revise a few things about bonding in organic molecules. Want to join the conversation? This 109.5 o arrangement gives tetrahedral geometry (figure 4). Mix at least 2 nonequivalent atomic orbitals (e.g.s and p). Then, compare the model to real molecules! Web here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Send feedback | visit wolfram|alpha. Web hybridization increases the overlap of bonding orbitals and explains the molecular geometries of many species whose geometry cannot be explained using a vsepr. Want to join the conversation? An atom has a given hybridization depending on the number of bonds extending from it. Web hybridization increases the overlap of bonding orbitals and explains the molecular geometries of many species whose geometry cannot be explained using a vsepr approach. This type of hybridization is required whenever an atom is surrounded by two groups of. Send feedback | visit wolfram|alpha. Hybridisation and geometry of molecules play a vital role in their reactivity. How does molecule shape change with different numbers of bonds and electron pairs? Sp 3 hybrid orbitals are oriented at bond angle of 109.5 o from each other. There is also an implicit geometric shape associated with the hybridization. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Ways carbon can bond to others. Reactions involve making and breaking of bonds! Web hybridization increases the overlap of bonding orbitals and explains the molecular geometries of many species whose geometry cannot be explained using a vsepr approach. Draw lewis structure,. One of the s orbital electrons is promoted to the open p orbital slot in the carbon electron configuration and then all four of the orbitals become hybridized to a uniform energy level as 1s + 3p = 4 sp 3 hybrid orbitals. Sp 2 hybridization results in trigonal geometry. Web sp³, sp² and sp hybridization, or the mixing of. Added may 3, 2012 by alessandroserpi in chemistry. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Find out by adding single, double or triple bonds and lone pairs to the central atom. One of the s orbital electrons is promoted to the open p orbital slot in the carbon. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Want to join the conversation? Web the hybridization of an atom is determined based on the number of regions of electron density that surround it. Explore molecule shapes by building molecules in 3d! Web hybridization increases the overlap of bonding orbitals and explains the molecular geometries of many species whose geometry cannot be explained using a vsepr approach. Add these two numbers together. Here is a chart that sums this up: Web summary vsepr and hybridization table. This 109.5 o arrangement gives tetrahedral geometry (figure 4). There is also an implicit geometric shape associated with the hybridization. Get the free hybridization widget for your website, blog, wordpress, blogger, or igoogle. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. The angle between the orbitals is 180°. Covalent bonds are formed by: Sp 2 hybridization results in trigonal geometry.
Hybridization Wize University Chemistry Textbook Wizeprep
What is Hybridization? sp3, sp2, Examples and Formula
.jpg)
Types of Hybridization Definitions, Examples, Key Features, Steps to
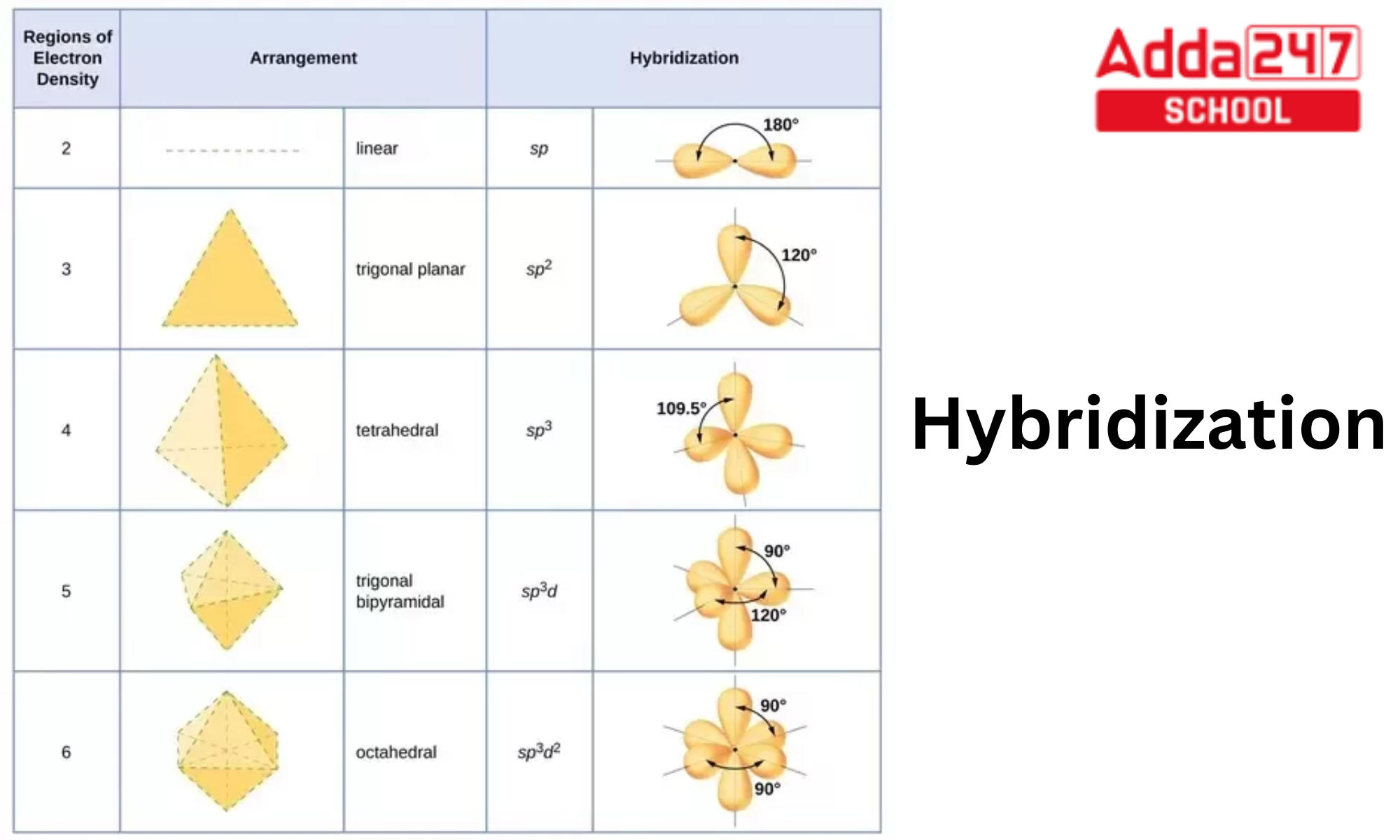
Hybridization Orbitals Chart

Molecular Geometry Chart With Hybridization carthopde
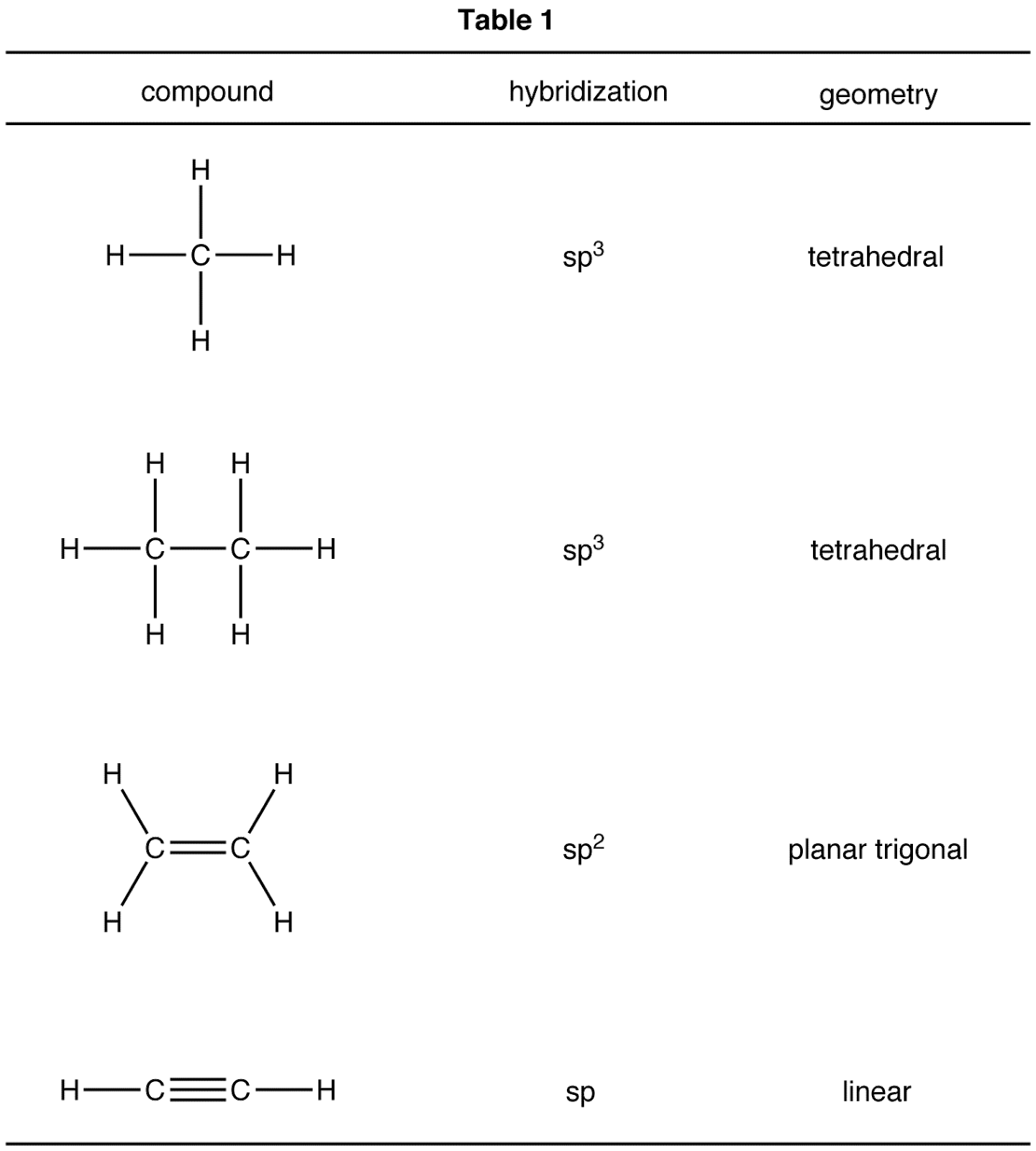
Molecular Geometry And Hybridization Chart
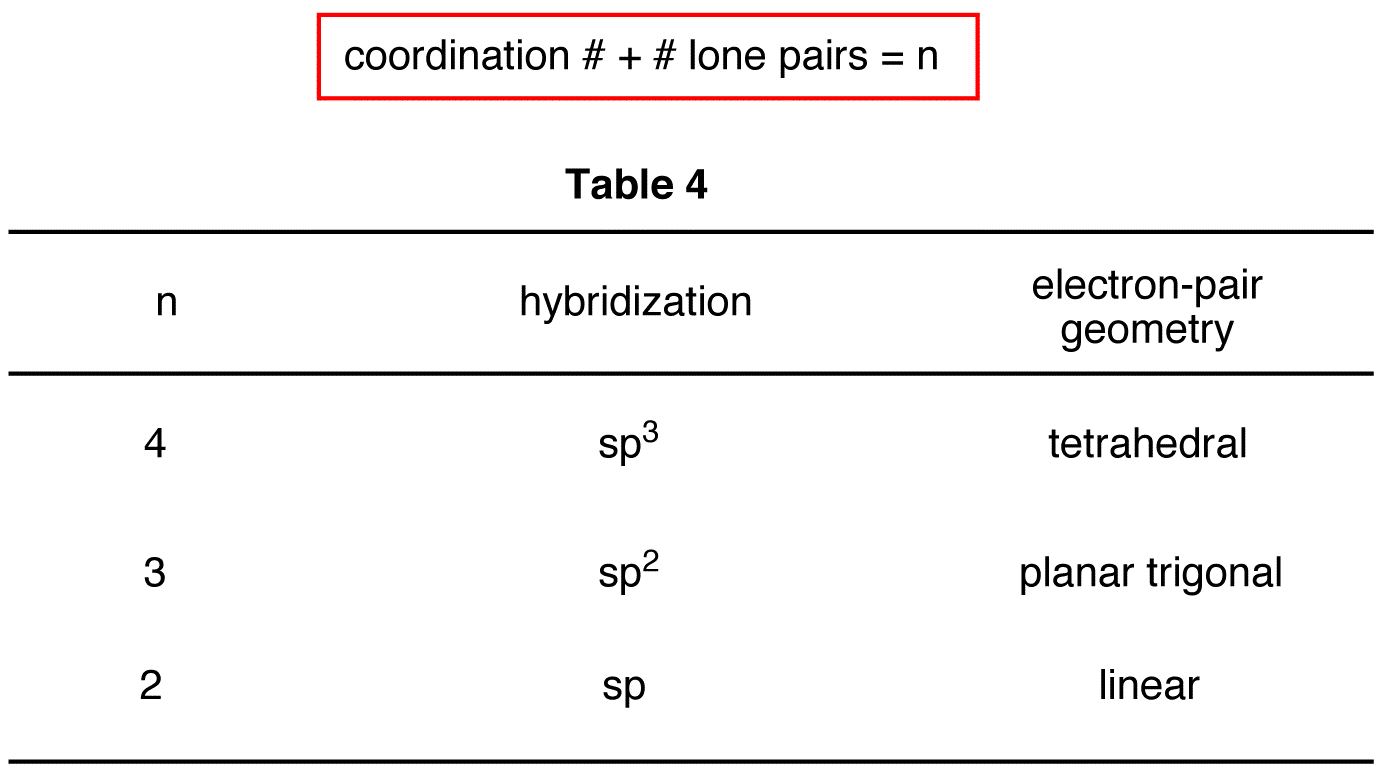
MariePreAPChem Hybridization

XeCl4 Lewis Structure, Geometry, Hybridization, and Polarity

Hybridization and Hybrid Orbitals ChemTalk

Molecular Geometry Chart With Hybridization
Web Here’s A Shortcut For How To Determine The Hybridization Of An Atom In A Molecule That Will Work In At Least 95% Of The Cases You See In Org 1.
Web The Five Basic Shapes Of Hybridization Are Linear, Trigonal Planar, Tetrahedral, Trigonal Bipyramidal And Octahedral.
Find Out By Adding Single, Double Or Triple Bonds And Lone Pairs To The Central Atom.
Sp 3 Hybrid Orbitals Are Oriented At Bond Angle Of 109.5 O From Each Other.
Related Post: