How To Draw Hydrogen
How To Draw Hydrogen - Web draw the dot and cross diagram with the outer shells overlapping. Web drawing organic molecules using lewis structures involves showing all atoms and bonds. Each episode dives into the latest trends, technologies, and policies shaping the future of hydrogen production, investment, infrastructure, and market dynamics. For larger molecules, this approach becomes increasingly cumbersome. In the hydrogen bohr model, a nucleus with 1 proton and 0 neutrons resides at the center. This is the only series of lines in the electromagnetic spectrum that lies in the visible region. Bohr's model does not work for systems with more than one electron. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Give each atom connected to the chiral center a priority based on its atomic number. Then draw the shared electrons inside the overlapping section. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch Try out different models by shooting light at the atom. The balmer series is basically the part of the hydrogen emission spectrum responsible for the. To hydrogenate an alkene, you need hydrogen gas and a metal catalyst, something like platinum or palladium or nickel. You. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. In april, the coalition for better schools, a community group, sent a survey asking residents if they were in favor of a name change. Connect the atoms to each other with single bonds to form a. E ( n) = − 1 n 2 ⋅ 13.6 ev. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. With a firm grasp on these. Give each atom connected to the chiral center a priority based on its atomic number. Bohr's model of hydrogen is based on the nonclassical. Web a very simple drawing of an hydrogen atom with a nucleus and an electron :) Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. The balmer series is basically the part of the hydrogen emission spectrum responsible for the. Then draw the shared electrons inside the overlapping section. Since. Try out different models by shooting light at the atom. Drawing on the expertise of… So, i'm gonna draw this around the carbon hydrogen bonds so we're. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web this series of the hydrogen emission spectrum is known as the balmer series. Kekulé formulas or structural formulas display the atoms of the molecule in the order they are bonded. In the hydrogen bohr model, a nucleus with 1 proton and 0 neutrons resides at the center. Drawing on the expertise of… We’ll use a bohr diagram to visually represent where the electrons. For example, ethanoic acid would be shown in a. The higher the atomic number, the higher the priority. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Surrounding this nucleus is a single electron shell, housing 1 electron. The nrma will install two of the. The number of dots equals the number of valence electrons in the atom. The bond between the two nitrogen atoms is a triple bond. Nitrogen gas occurs naturally as a diatomic molecule. And there are many others, but these are the ones most commonly used. [1] to draw the hydrogen bohr model, begin by noting the 1 proton and 0. You will also explore the role of water in biological systems and its importance for life on earth. Create the nucleus, and then proceed to sketch the. Check how the prediction of the model matches the experimental results. Then draw the shared electrons inside the overlapping section. So, we leave those out in bond line structures. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. So,. Drawing chemical structures is shared under a license and was authored, remixed, and/or curated by steven farmer, dietmar kennepohl, krista cunningham, tim soderberg, william reusch, & william reusch. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. We’ll use a bohr diagram to visually represent where the electrons. This video shows three examples of drawing for the formation of hydrogen bond. Give each atom connected to the chiral center a priority based on its atomic number. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Check how the prediction of the model matches the experimental results. So, i'm gonna draw this around the carbon hydrogen bonds so we're. The higher the atomic number, the higher the priority. To hydrogenate an alkene, you need hydrogen gas and a metal catalyst, something like platinum or palladium or nickel. Bohr's model does not work for systems with more than one electron. These dots are arranged to the right and left and above and below. Then draw the shared electrons inside the overlapping section. The bond between the two nitrogen atoms is a triple bond. Web drawing organic molecules using lewis structures involves showing all atoms and bonds. [1] to draw the hydrogen bohr model, begin by noting the 1 proton and 0 neutrons.
How to Draw the Lewis Dot Structure for H+ (Hydrogen ion) YouTube

Hydrogen Atom Diagram

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
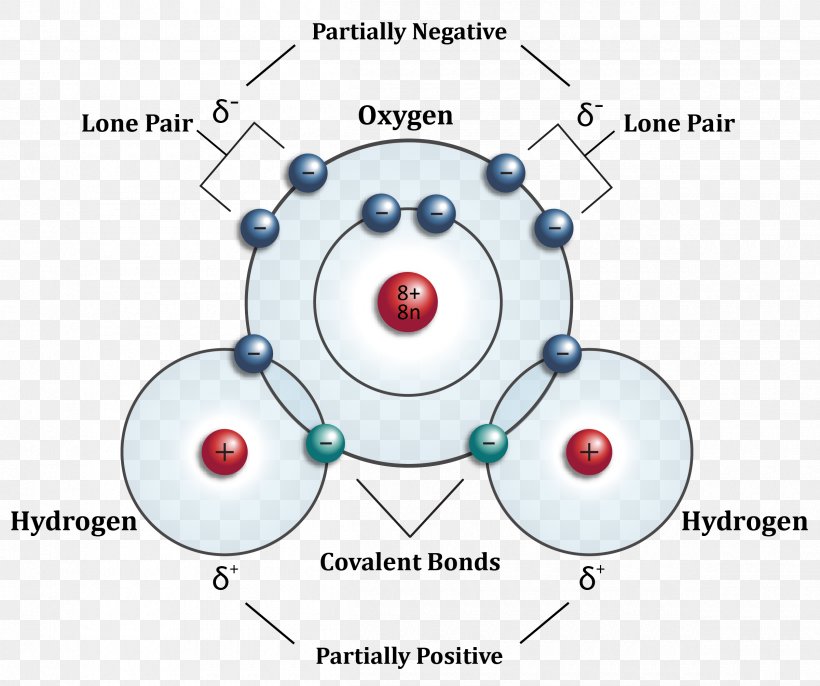
Hydrogen Molecule Structure
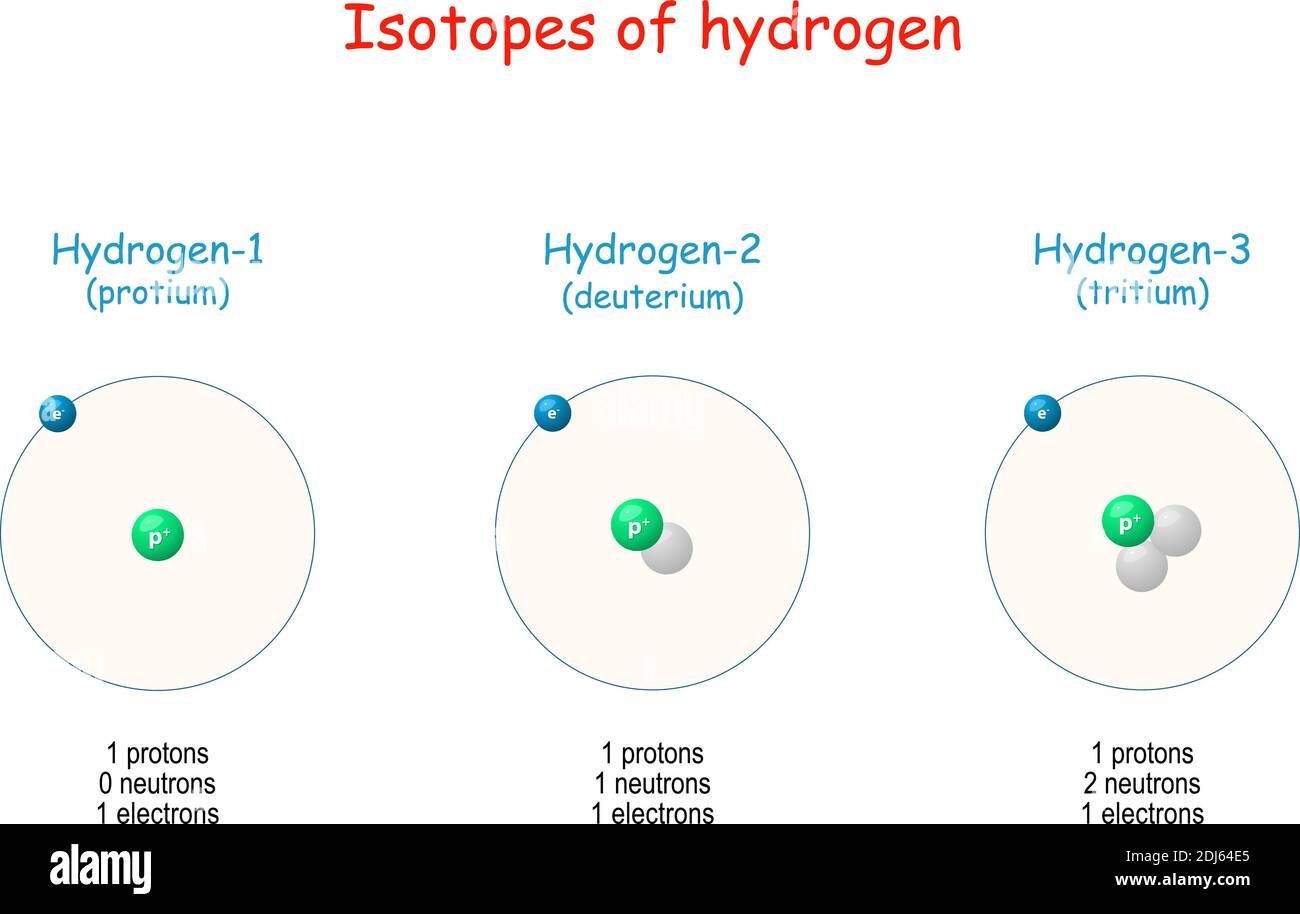
Atomic Structure Of Hydrogen

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
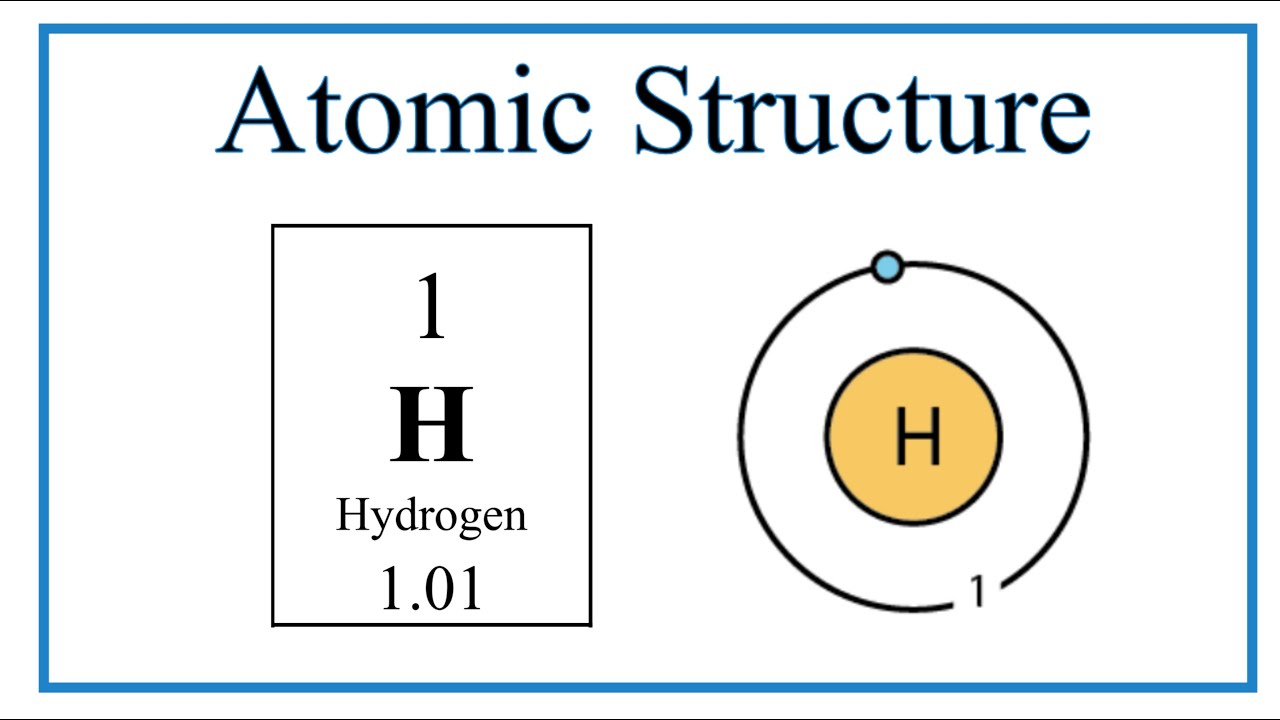
Atomic Structure (Bohr Model) for Hydrogen (H) YouTube

How To Draw Hydrogen Bonds Askworksheet

Diagram Representation Of The Element Hydrogen Stock Vector Image

Diagram representation element hydrogen Royalty Free Vector
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Drawing On The Expertise Of…
Since Each Carbon Requires 4 Bonds, The Number Of Hydrogen Atoms Can Be Determined By The Existing Number Of Bonds In The Molecule.
When Constructing A Lewis Diagram, Keep In Mind The Octet Rule, Which Refers To The Tendency Of Atoms.
Related Post: