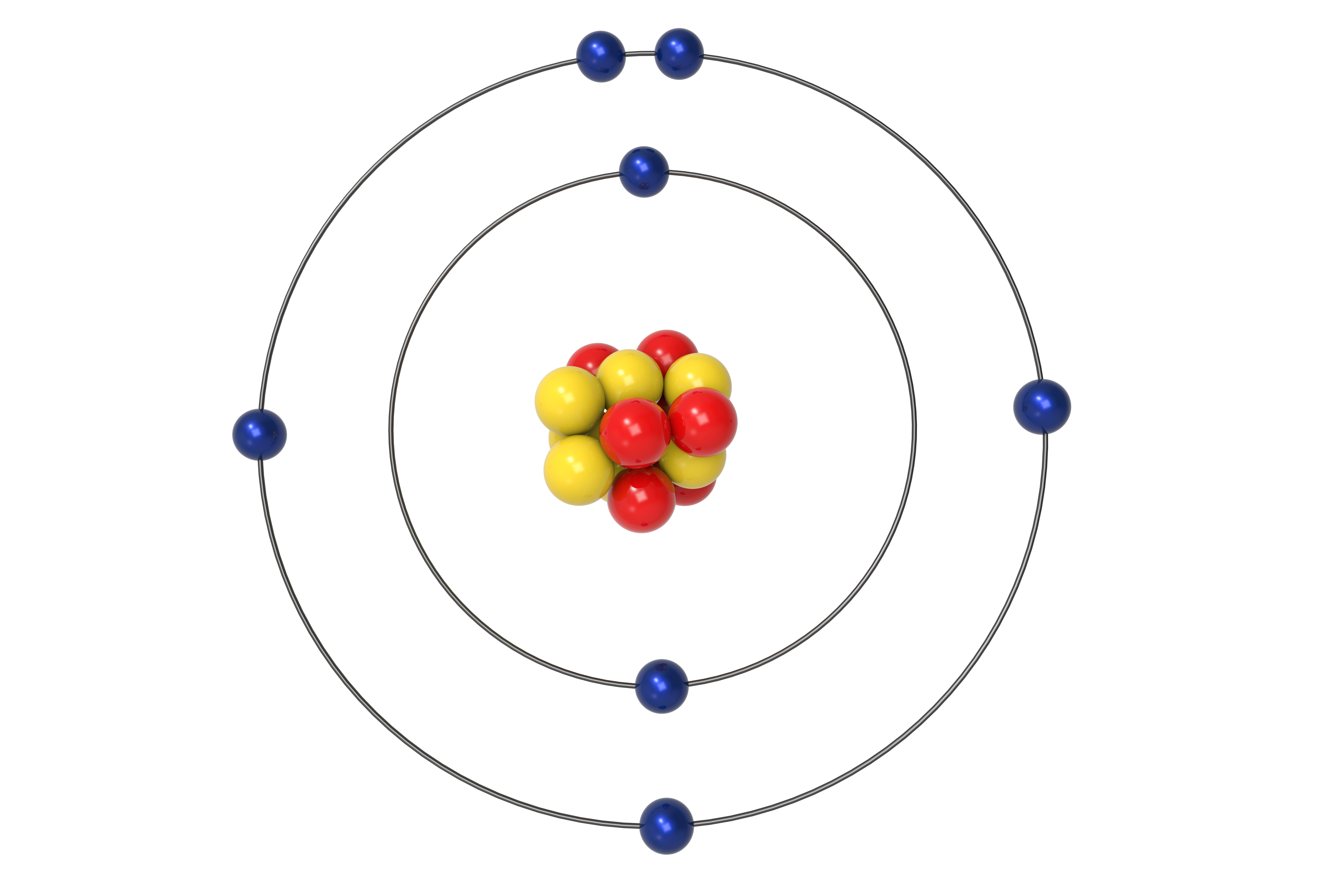
Electrons Drawing
Electrons Drawing - Use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. Web a simpler method has been proposed for constructing lewis structures, eliminating the need for electron counting: Web drawing on extensive previous studies of neural connections in worms by leifer’s team and fruit flies by murthy’s team, the two scientists and their teams will develop a system to create maps of functional connectivity that will shed light on how the brain controls activities such as decision making and movement. Electrons must occupy the lowest available shell, closest to the nucleus. Web steps to drawing a lewis structure. Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Four of them fill the 1s and 2s orbitals. Web here's some of the guidelines for drawing dot structures. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electron configurations describe where electrons are located around the nucleus of an atom. Four of them fill the 1s and 2s orbitals. Web here are the steps to draw a lewis structure. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Electrons must occupy the lowest available shell, closest to the nucleus. Carbon (atomic number 6) has six electrons. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s. Lone pairs, unpaired electrons, and. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. The example is for the nitrate ion. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Shared pairs of electrons are. This indicates not only that they are electrons, but helps remind the viewer that electrons contain a negative charge. Web here's some of the guidelines for drawing dot structures. This will be the atom with the lowest electronegativity. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. The atoms are drawn showing. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Web construct an atom according to the bohr model. Four of them fill the 1s and 2s orbitals. This will be the atom with the lowest electronegativity. Want to join the conversation? Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. For that, we have electron shell diagrams. Lone pairs, unpaired electrons, and. Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration diagrams. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Electron configurations describe where electrons are located around the nucleus of an atom. (hydrogen is excluded because it. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web build an. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. And we would account for these valence electrons in our dot structure. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. The atoms are drawn showing the valence. Web valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration diagrams. The remaining two electrons occupy the 2p subshell. For example, the electron configuration of lithium, 1s²2s¹, tells. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration diagrams. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A simple tutorial on how. Web the best way to draw electrons is to draw them as circles with minus signs inside. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Electrons must occupy the lowest available shell, closest to the nucleus. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Web steps to drawing a lewis structure. The atoms are drawn showing the valence electrons; Four of them fill the 1s and 2s orbitals. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The example is for the nitrate ion. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. And we would account for these valence electrons in our dot structure. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Web construct an atom according to the bohr model. Web updated on november 05, 2019. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.![Distribution of Electrons in Different Orbits [with Examples] Teacho](https://d1avenlh0i1xmr.cloudfront.net/00d8e8eb-2904-4147-abf9-6d87a6c24f05/14.-orbits-teachoo-01.png)
Distribution of Electrons in Different Orbits [with Examples] Teacho
Lets Get Inside An Atom!! The Science Station
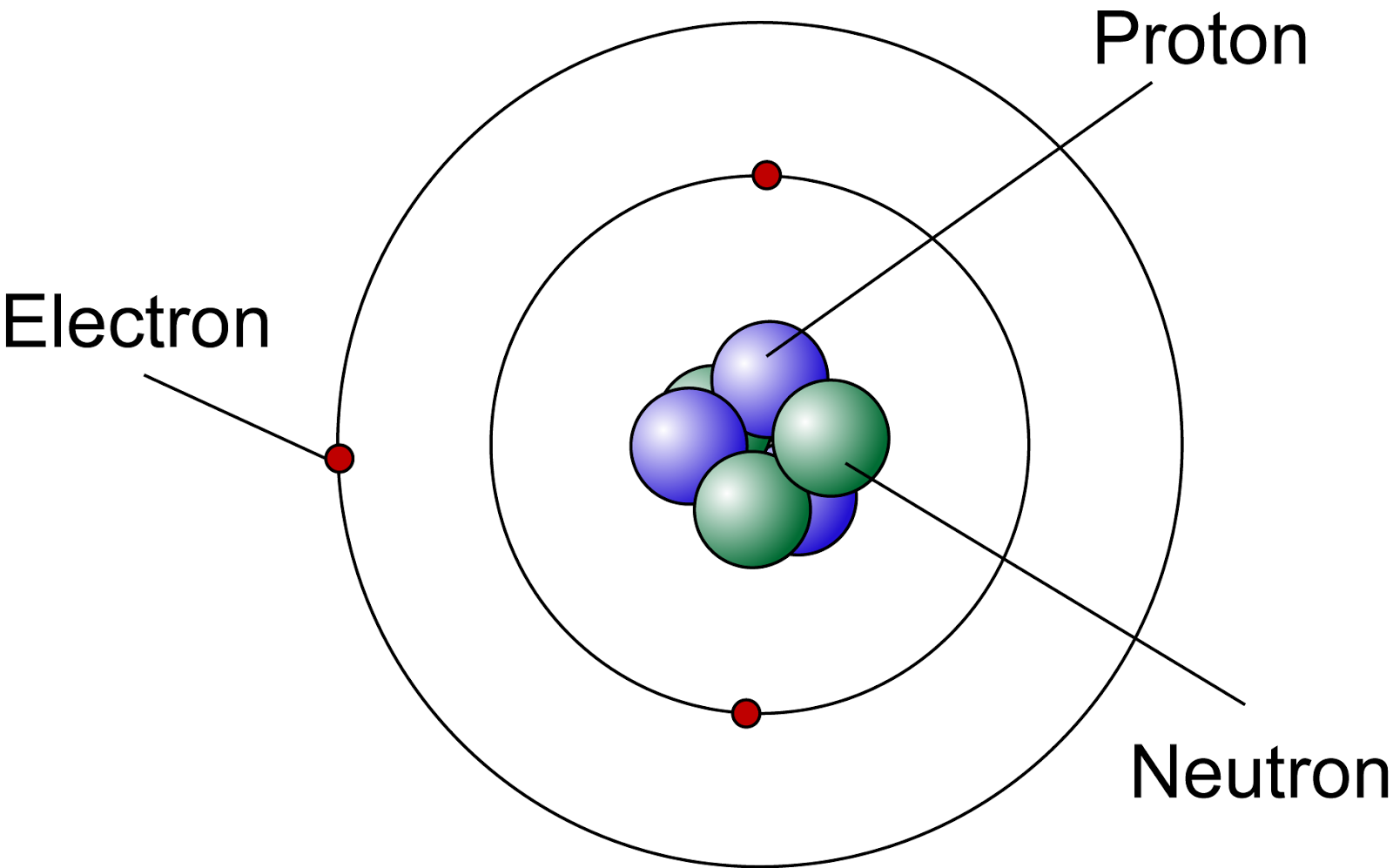
Label The Parts Of A Atom
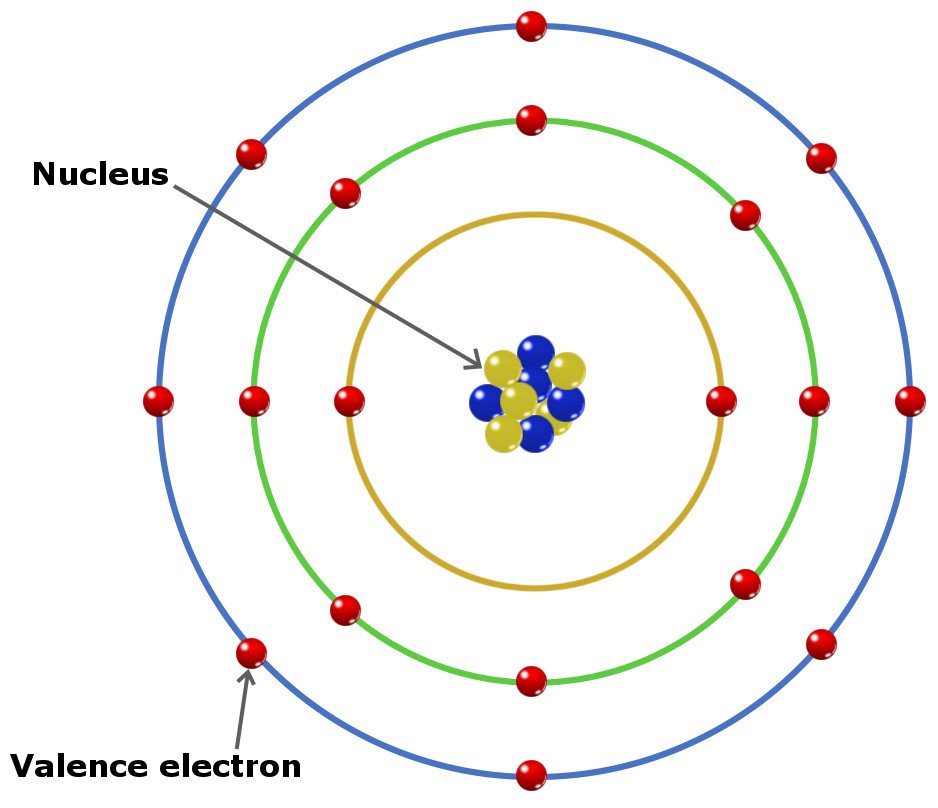
What Are Valence Electrons And How To Find Them? Where Are They Located?
Electrons Biology for Majors I
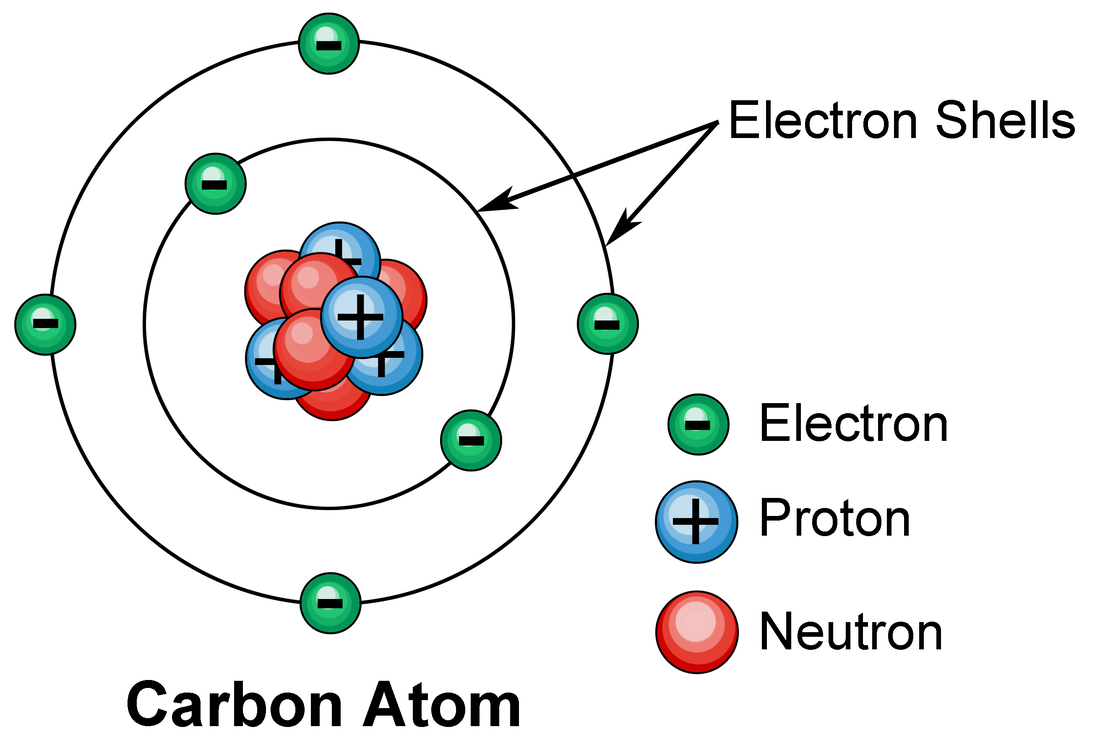
Electronic structure of matter. San Francisco de Paula, Science
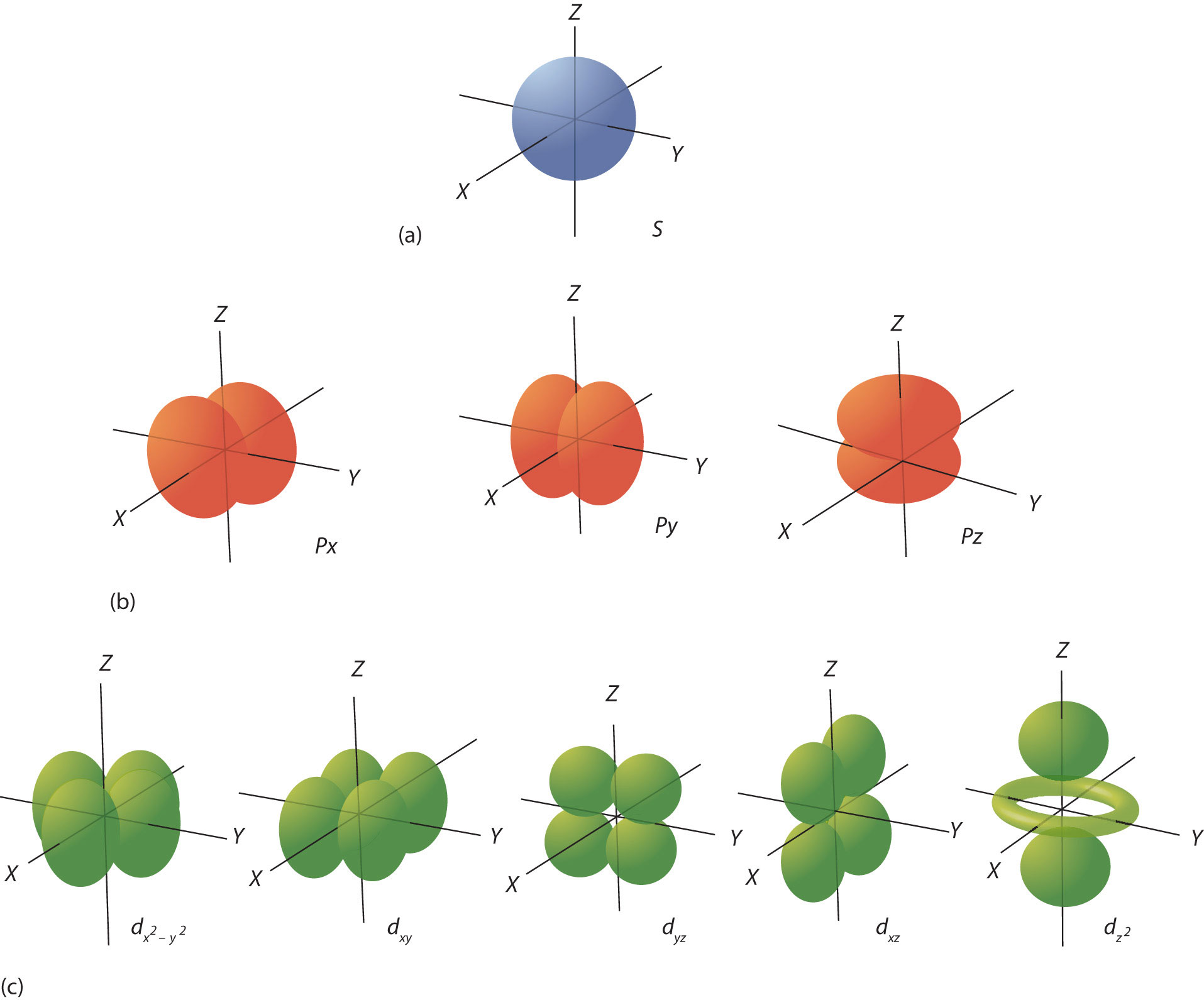
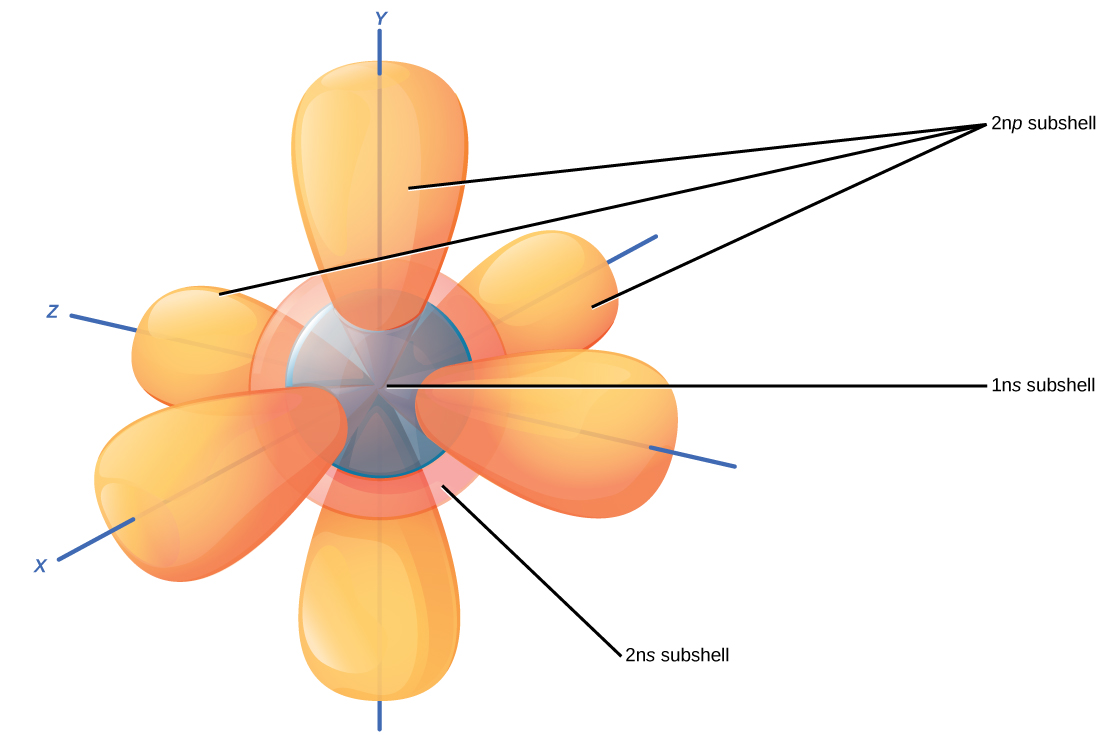
2.3 Quantum Numbers for Electrons Chemistry LibreTexts
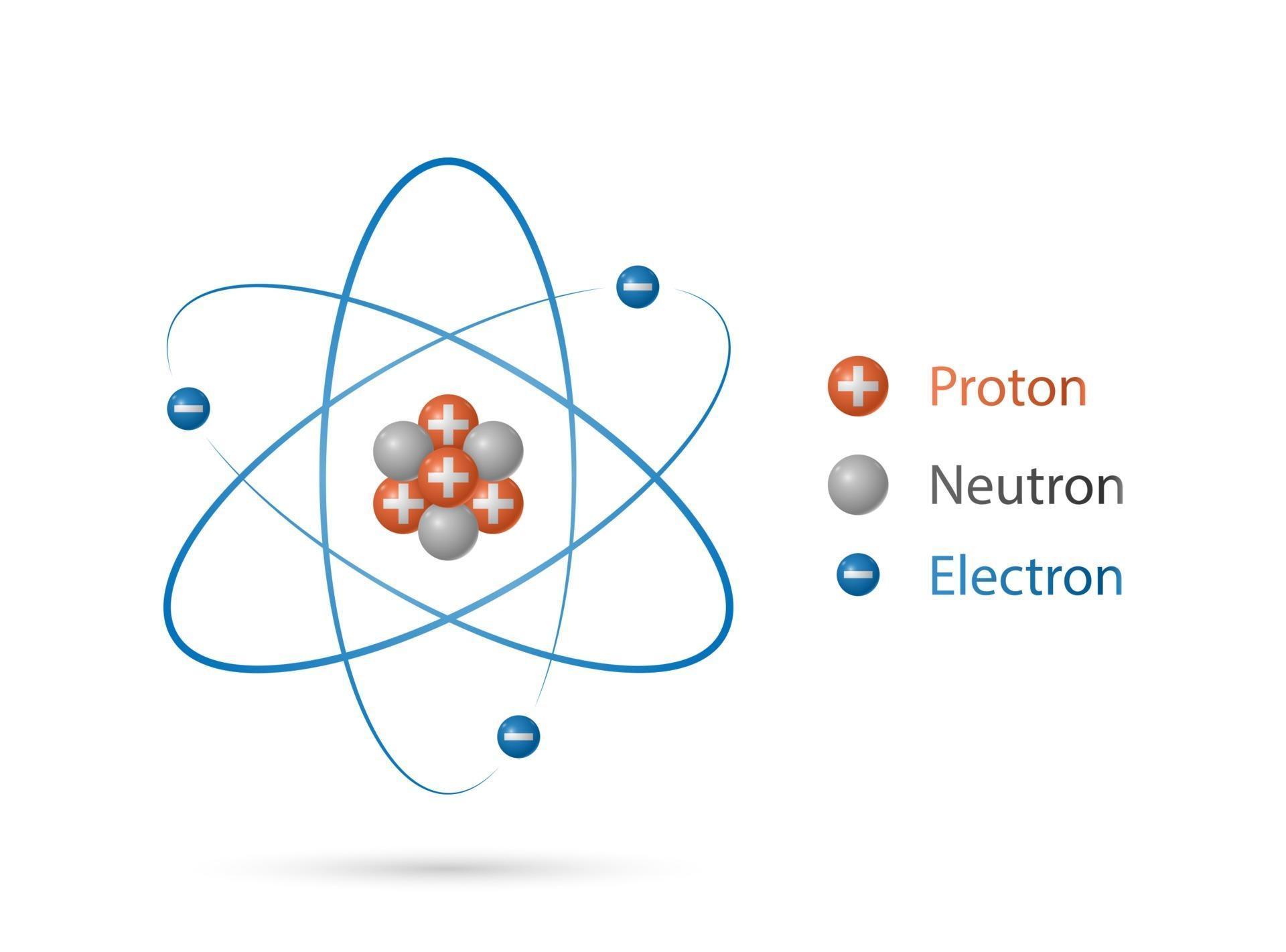
modelo de estructura de átomo, núcleo de protones y neutrones

Atomic Structure Broad Learnings
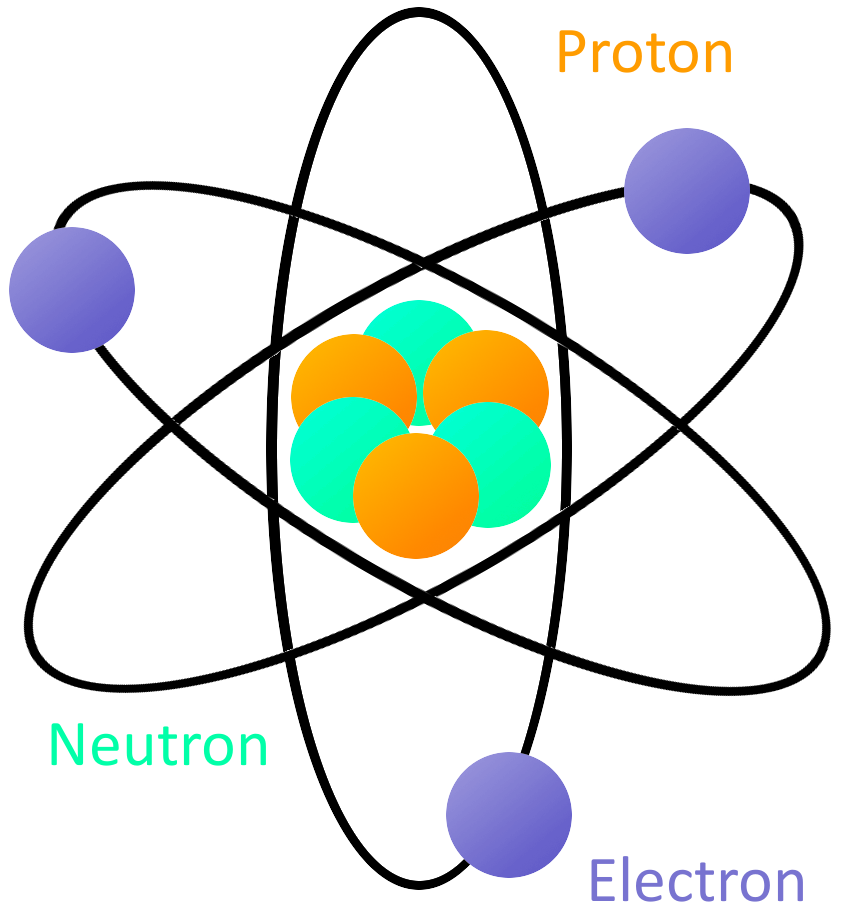
What is Electricity? SparkFun Learn
So Let's Say We Wanted To Draw The Dot Structure For This Molecule, So Silicon Tetrafluoride.
To Facilitate Our Understanding Of How Valence Electrons Interact, A Simple Way Of Representing Those Valence Electrons Would Be Useful.
Electron Configurations Describe Where Electrons Are Located Around The Nucleus Of An Atom.
Take Sodium As An Example.
Related Post: