Draw The Structure Of Aniline
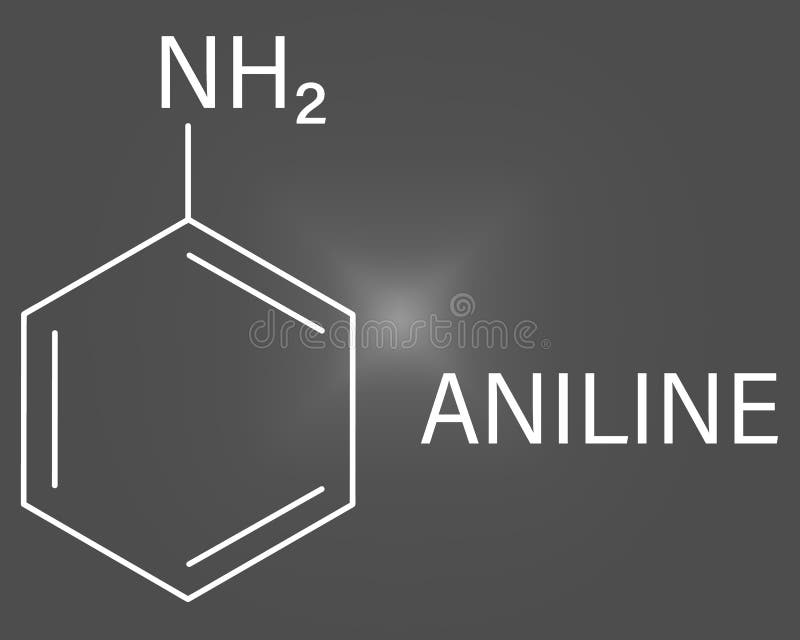
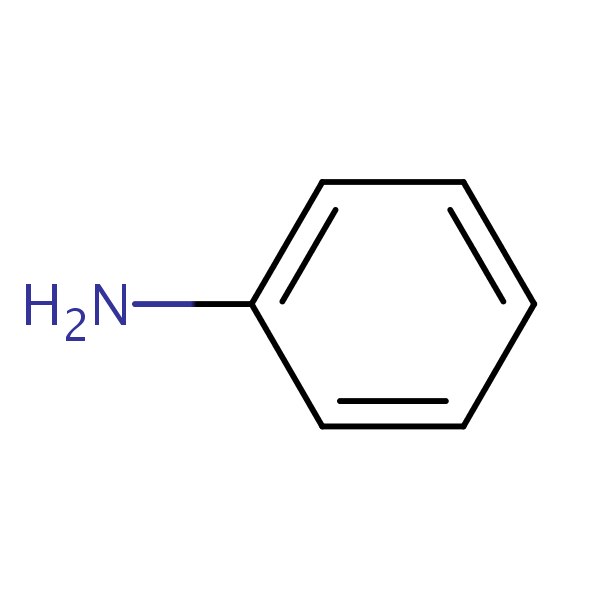
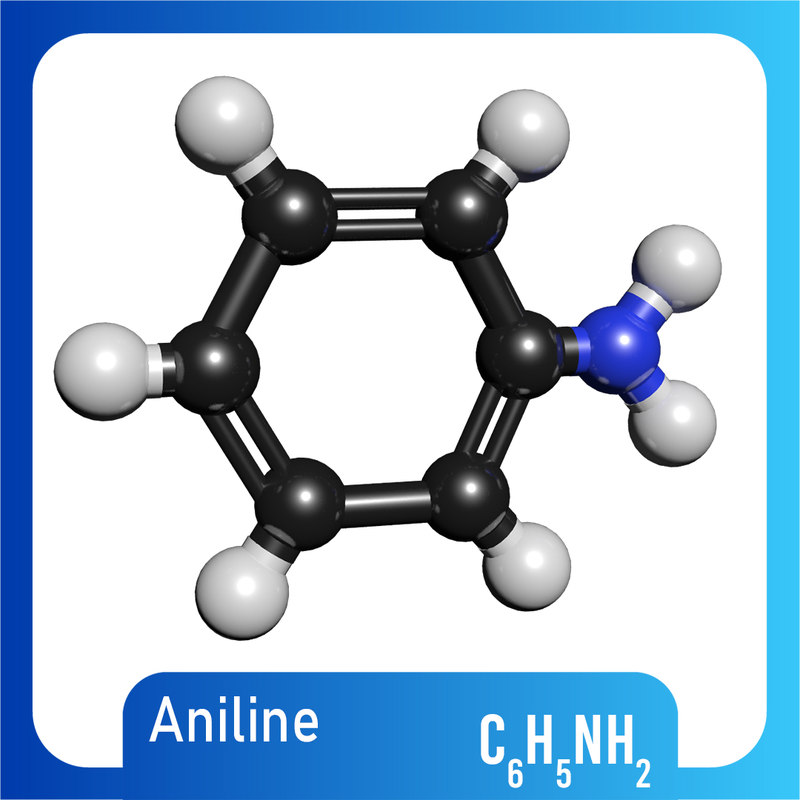
Draw The Structure Of Aniline - Aniline, also known as aminobenzene or phenylamine, has 6 carbon (c) atoms, 7 hydrogen (h) atoms, and 1 nitrogen (n) atom in its chemical formula of c6h7n or c6h5nh2. This results in formation of a double bond between c and n with n getting a positive charge due to the donation of electrons. This salt then undergoes various nucleophilic substitution reactions. Web #k2chemistryclass #resonance #aniline #aniline_resonance #resonance_structure #chemistryformula #chemistry #compound #chemicalformula #science #rasayansha. The compound is a derivative of aniline. This results in the chemical formula c 6 h 5 nh 2. Web the structure of phenylamine; Web as this degradation reaction essentially indicates the removal of one aniline molecule from the tam structure, a shift to irregular and larger pores can be expected. Draw the structure of aniline. Draw the structure of aniline. 1 n atom joined to benzene ring. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Each c atom is bonded with one h atom. Its chemical formula is c 6 h 5 nh 2 or c 6 h 7 n. It has hybridization of the nitrogen which is between \(sp_3\) and \(sp_2\). Web this structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. A lone pair of electrons on n atom. Web aniline is diazotized to give a diazonium salt. Web the structure of phenylamine; The resonance structures of aniline are drawn by first displacing. Click here:point_up_2:to get an answer to your question :writing_hand:draw the resonating structure of aniline. As an amine, aniline has a lone pair of electrons on the nitrogen atom that can participate in a wide range of chemical reactions, enabling it to serve as a precursor for many valuable. It has hybridization of the nitrogen which is between \(sp_3\) and \(sp_2\).. This structure is also available as a 2d mol file or as a computed 3d sd file. A lone pair of electrons on n atom. Since it consists of 6 carbon atoms, 7 hydrogen atoms, and 1 nitrogen atom in its chemical formula, it is an organic compound. It has hybridization of the nitrogen which is between \(sp_3\) and \(sp_2\).. Still, the number of degraded linkers should be limited, as the effect on the crystallinity is minor. 1 n atom joined to benzene ring. Consisting of a phenyl group (−c 6 h 5) attached to an amino group (−nh 2), aniline is the simplest aromatic amine. Draw the structure of aniline. Since it consists of 6 carbon atoms, 7 hydrogen. Web find the formula and structure of aniline. A lone pair of electrons on n atom. This problem has been solved! C 6 h 7 n. Web this structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web this structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Weast and grasselli, 1989 crc. The resonance structures of aniline are drawn by first displacing the. Web the compound of aniline is soluble in water which is colourless to light brown. Web there are four (technically five) resonance structures for aniline. As an amine, aniline has a lone pair of electrons on the nitrogen atom that can participate in a wide range of chemical reactions, enabling it to serve as a precursor for many valuable. It. Aniline is substantially less basic than methylamine, as is evident by looking at the pk a values for their respective ammonium conjugate acids (remember that the lower the pka of the conjugate acid, the weaker the base). Observed bonds and angles are illustrated on the central drawing of the. A lone pair of electrons on n atom. Still, the number. This structure is also available as a 2d mol file or as a computed 3d sd file. Consisting of a phenyl group (−c 6 h 5) attached to an amino group (−nh 2), aniline is the simplest aromatic amine. And a determination of the specific heats of aniline and benzene over the approximate range 20°c to 50°c, proc. Web this. Here i show how to create them all by pushing electrons around.check me out: This problem has been solved! Web there are four (technically five) resonance structures for aniline. Web this structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Web the compound of aniline is soluble in water which is colourless to light brown. It has hybridization of the nitrogen which is between \(sp_3\) and \(sp_2\). Consisting of a phenyl group (−c 6 h 5) attached to an amino group (−nh 2), aniline is the simplest aromatic amine. A lone pair of electrons on n atom. Each c atom is bonded with one h atom. Draw the structure of aniline. Web #k2chemistryclass #resonance #aniline #aniline_resonance #resonance_structure #chemistryformula #chemistry #compound #chemicalformula #science #rasayansha. Web find the formula and structure of aniline. Click here:point_up_2:to get an answer to your question :writing_hand:draw the resonating structure of aniline. Still, the number of degraded linkers should be limited, as the effect on the crystallinity is minor. Web the structure of phenylamine; This structure is also available as a 2d mol file or as a computed 3d sd file.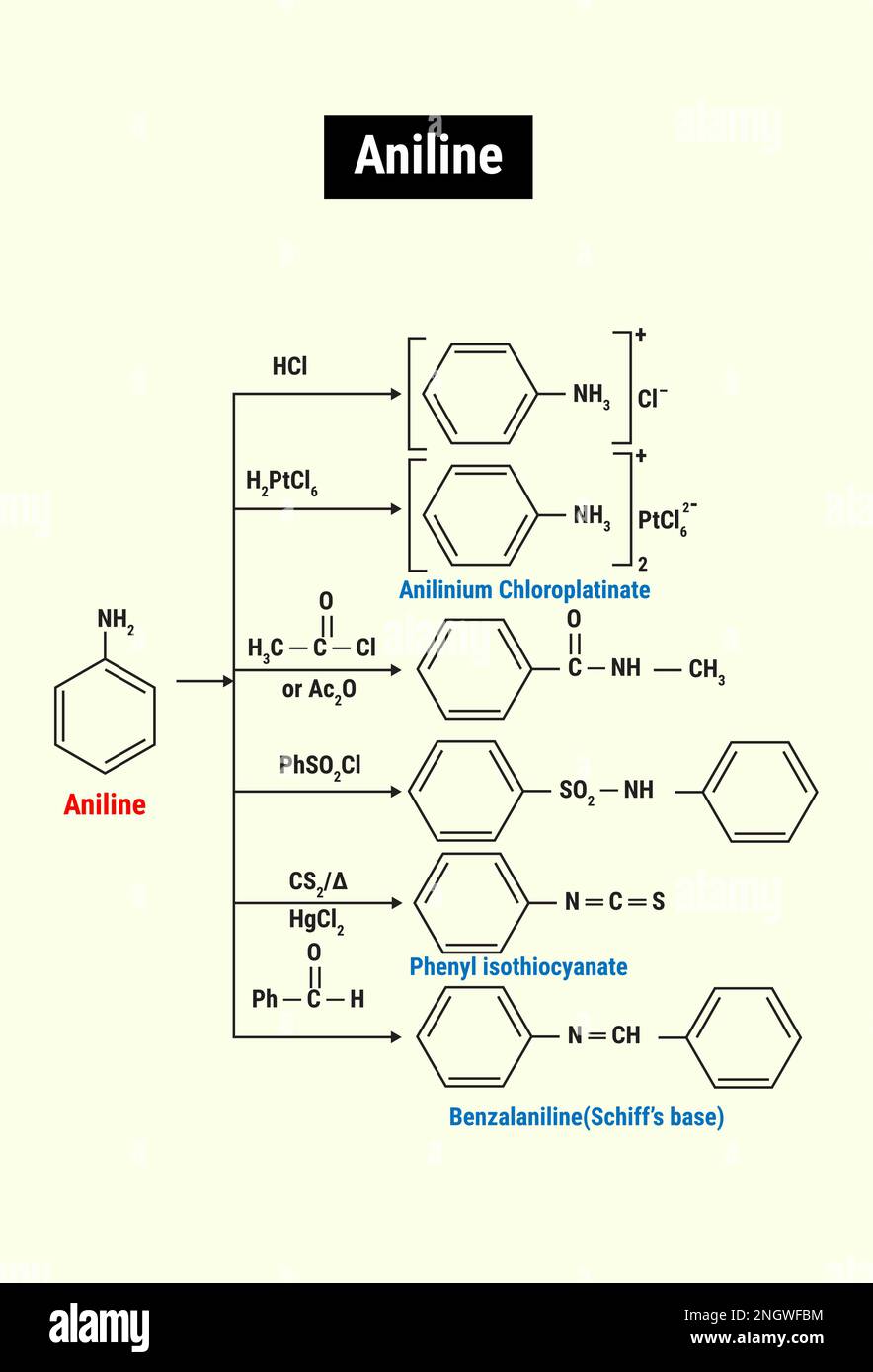
Chemical structure or reaction of Aniline Stock Vector Image & Art Alamy
Aniline Molecule. Also Known As Phenylamine, Aminobenzene. Skeletal
Draw the structure of aniline

Aniline stock vector. Illustration of pigment, paracetamol 42026736
Aniline SIELC
3D c6h5nh2 molecule aniline TurboSquid 1422230
Draw the structure of aniline

15.10 Amines Structures and Names Chemistry LibreTexts
Draw the structure of aniline

Chapter 17 Amines and Amides Chemistry 114 with Divis at Franciscan
Draw The Structure Of Aniline.
The Resonance Structures Of Aniline Are Drawn By First Displacing The Lone Pair Of Electrons On The Nitrogen To The Bond Between C And N.
C 6 H 7 N.
This Results In The Chemical Formula C 6 H 5 Nh 2.
Related Post: