Draw The Lewis Structure For The Ammonium Ion
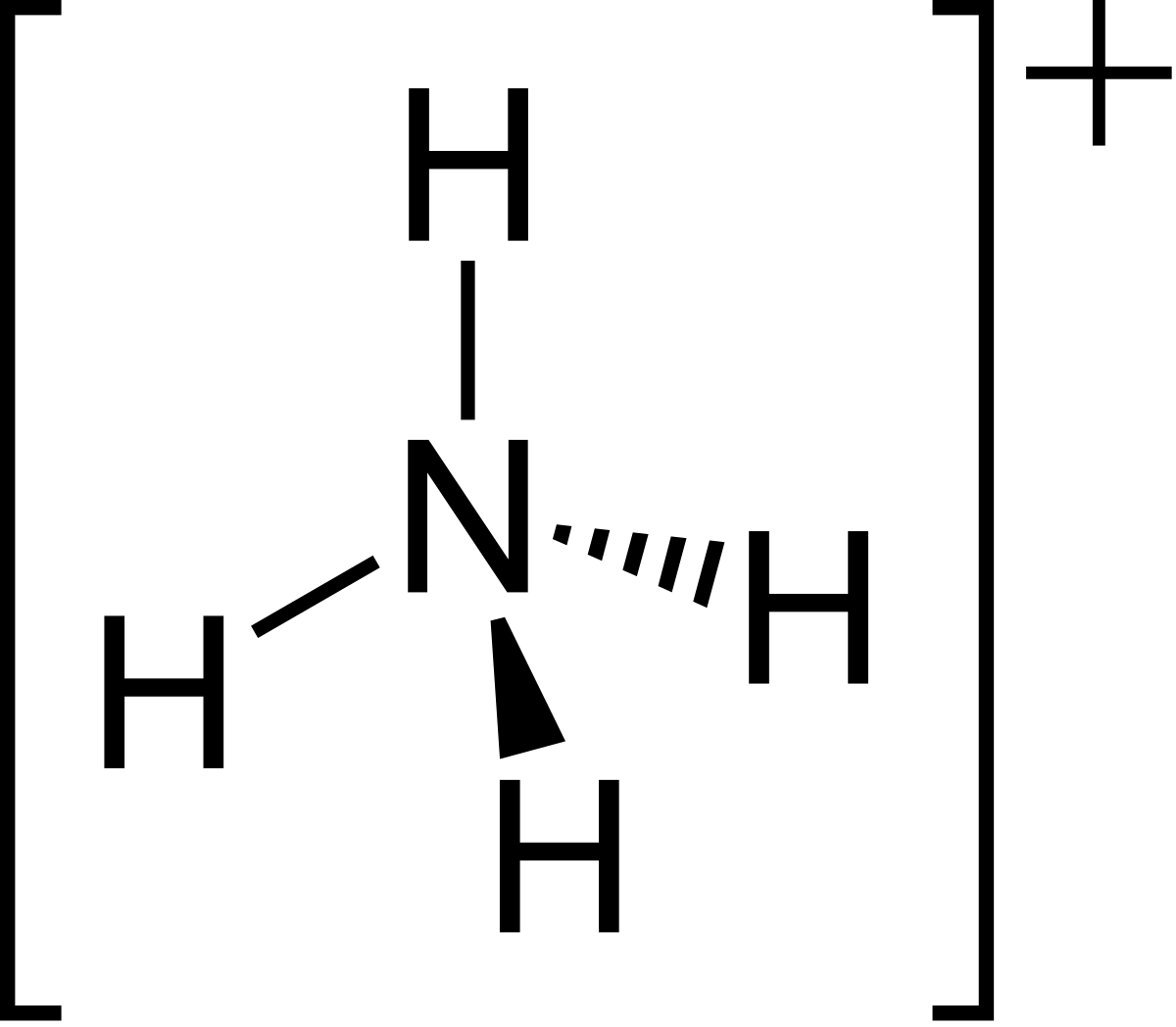
Draw The Lewis Structure For The Ammonium Ion - There is a +1 charge on nitrogen atom. Web when drawing the lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. Web draw the lewis structure for the ammonium ion (nh4+). Web the nh4+ ion, also known as the ammonium ion, is a common polyatomic cation in chemistry. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web let's do the lewis structure for nh4+, the ammonium ion. Web this video explains writing lewis structure of ammonium ion ( nh4+) in stepwise manner. This structure is commonly used in chemistry to depict the arrangement of electrons in a molecule or ion. Web this chemistry video tutorial explains how to draw the lewis structure of the ammonium ion nh4+. It is formed by the protonation of ammonia, nh3, and has a tetrahedral shape. Web lewis structure of nh4+, ammonium ion. Web in nh 4 + (ammonium ion) lewis structure, there are four sigma bonds around nitrogen atom. Calculate the total number of valence electrons. Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. There. Web a protonated ammonium ion or nh4+ is made up of nitrogen and hydrogen. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. Delving into the intricate world of chemistry, we embark on a. The exception, of course, being the hydrogen's. Steps of drawing the lewis structure of nh 4. How to draw lewis structures: A simple notation used to represent valence electrons in an atom is called lewis symbol. Web a brief explanation for how to write the lewis dot structure for the ammonium ion including it's molecular geometry and bond angles. Web this video explains writing lewis structure of ammonium ion ( nh4+) in stepwise manner. 1 n. Find more chemistry widgets in wolfram|alpha. Show the calculation of the formal charge on nitrogen and hydrogen in the ammonium ion. A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. Web lewis structure of nh4+, ammonium ion. For the nh4+ structure use the periodic table to find the total number of. They follow the duet rule (2 electrons). For the ammonium ion lewis structure, we first. In the case of the ammonium ion: Web the nh4+ lewis structure is a representation of the ammonium ion, which consists of four hydrogen atoms bonded to a central nitrogen atom. A simple notation used to represent valence electrons in an atom is called lewis. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web lewis dot of the ammonium ion. Nh3 + h+ ——> nh4+ It is formed by the protonation of ammonia, nh3, and has a tetrahedral shape. Web draw the lewis structure for the ammonium ion (nh4+). Web a protonated ammonium ion or nh4+ is made up of nitrogen and hydrogen. So we've lost one, that's minus one. Web this video explains writing lewis structure of ammonium ion ( nh4+) in stepwise manner. And if you see a plus sign, that means you've lost a valence electron. Steps of drawing the lewis structure of nh 4 +. This problem has been solved! Web in nh 4 + (ammonium ion) lewis structure, there are four sigma bonds around nitrogen atom. So we've lost one, that's minus one. Here’s the best way to solve it. Web 6 steps to draw the lewis structure of nh4+ step #1: Nh3 + h+ ——> nh4+ Web draw the lewis structure for the ammonium ion (nh4+). A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. Web in nh 4 + (ammonium ion) lewis structure, there are four sigma bonds around nitrogen atom. Get the free lewis structure finder widget for your website,. Web i quickly take you through how to draw the lewis structure of nh4+, (ammonium ion). Calculate the total number of valence electrons. Using the periodic table to draw lewis dot. Steps of drawing the lewis structure of nh 4 + are explained in this tutorial. Web draw the lewis structure for ammonium, nh4+, include formal charges. 4h 4 h atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons. Web draw the lewis structure for ammonium, nh4+, include formal charges. These atoms will combine with covalent. Show the calculation of the formal charge on nitrogen and hydrogen in the ammonium ion. Web when drawing the lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web a protonated ammonium ion or nh4+ is made up of nitrogen and hydrogen. So nitrogen, on the periodic table, is in group 5, so it has 5 valence electrons. Find more chemistry widgets in wolfram|alpha. Web draw the lewis structure for the ammonium ion (nh4+). Which sentence or sentences have correct parallel structure ixl answers: Four hydrogens, so let's multiply that times 4. For the ammonium ion lewis structure, we first. In the case of the ammonium ion: For the nh4+ structure use the periodic table to find the total number of valence electrons for the. Understanding its lewis structure is essential to understanding its properties and reactions.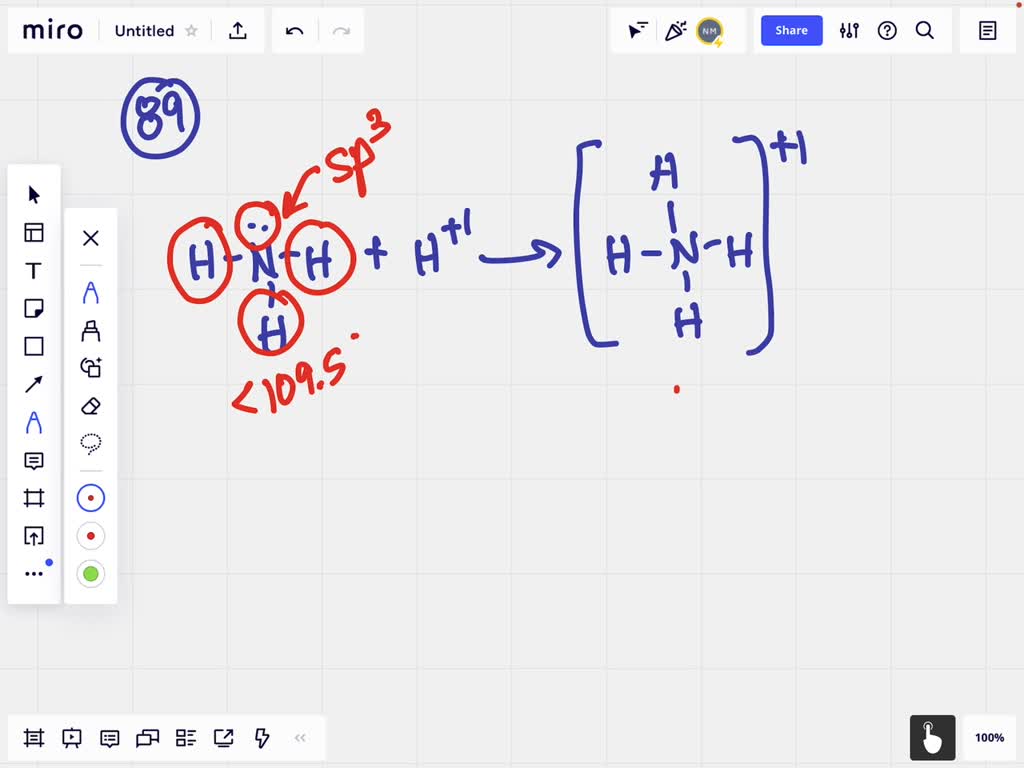
SOLVEDDraw the Lewis formula of an ammonium ion. Describe the
Lewis Dot Diagram Of Ammonia Wiring Diagram Pictures
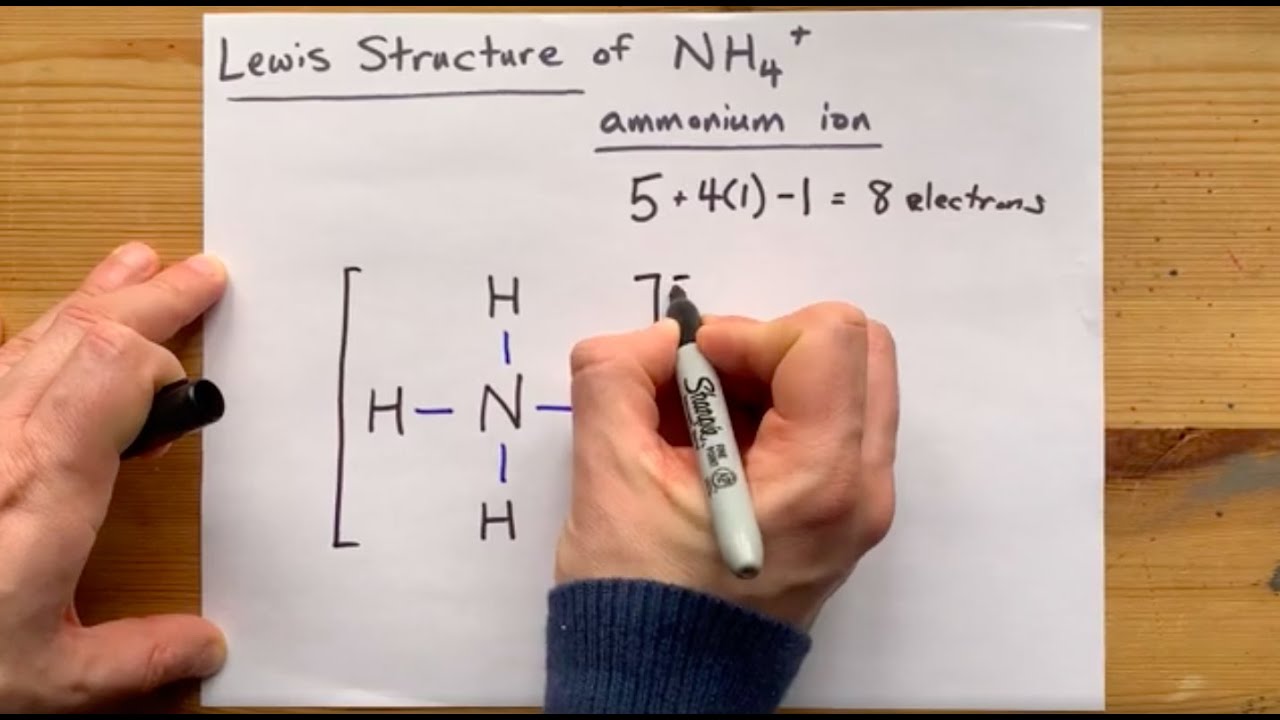
Lewis Structure of NH4+, Ammonium ion YouTube
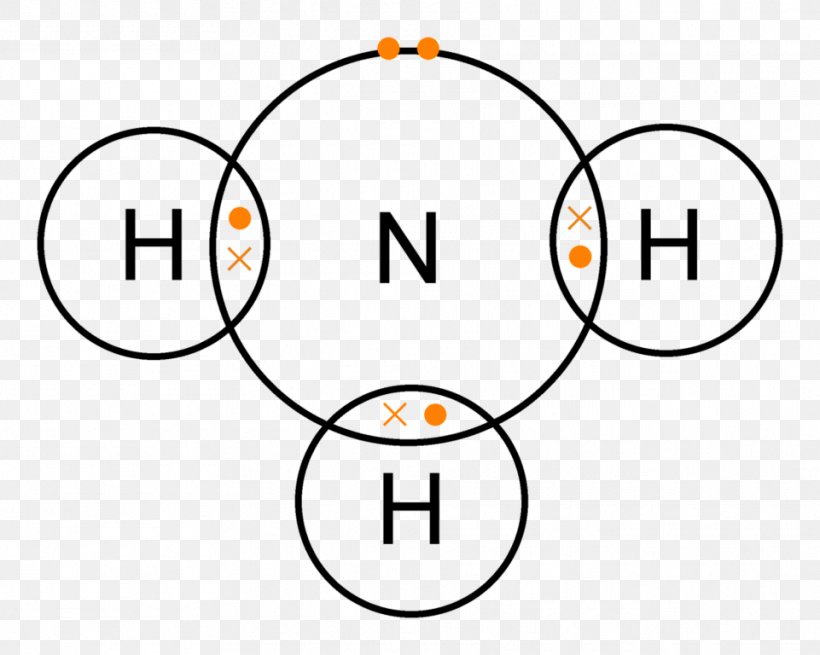
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG

Draw the Lewis structure for ammonium ion. Quizlet

NH4+ Lewis Structure (Ammonium Ion) in 2021 Lewis, Math equations, Nh 4
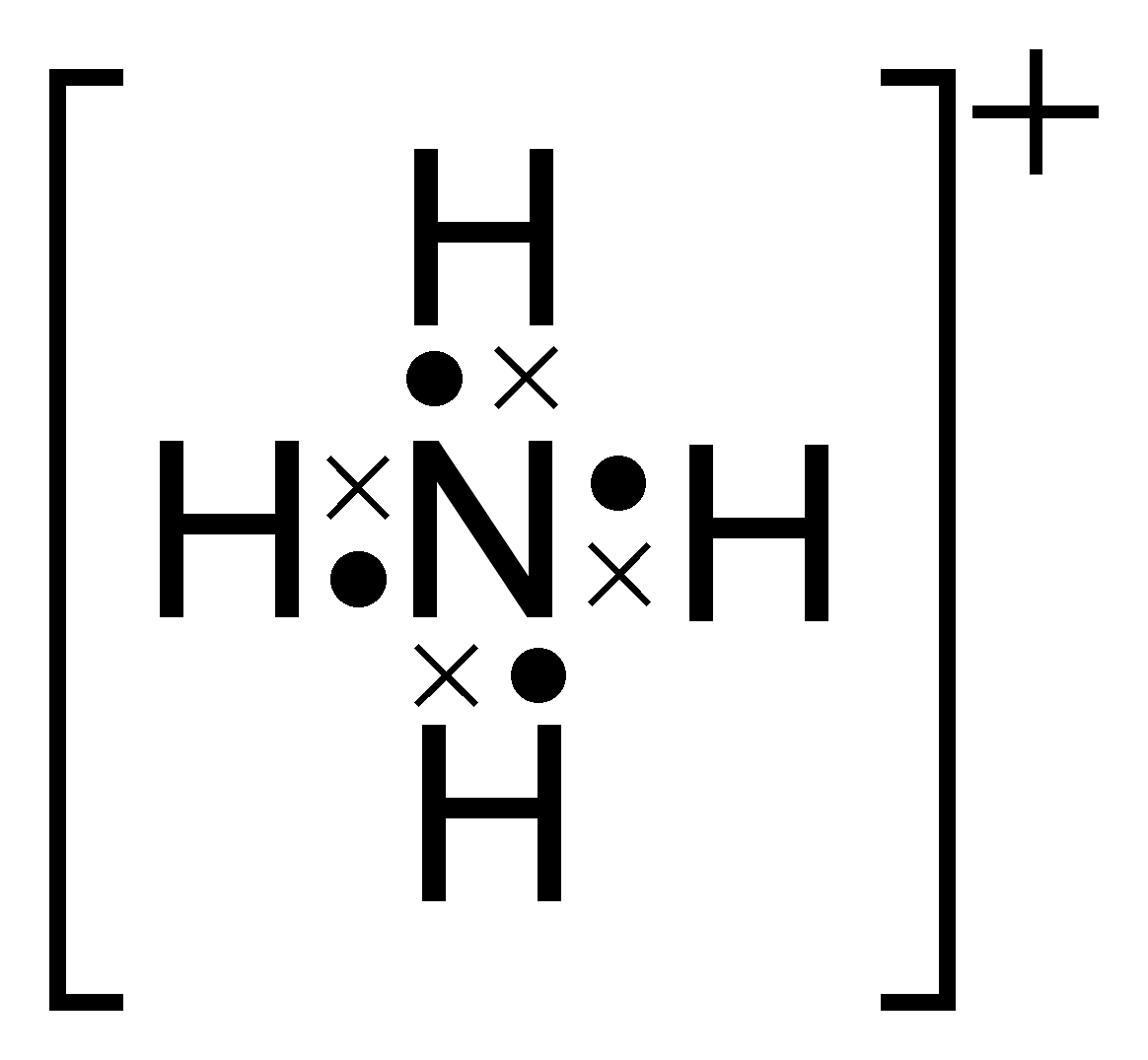
Electron Dot Diagram Of Ammonium Ion Wiring Diagram Pictures

CHEMISTRY 101 Drawing Lewis Structures polyatomic ions, ammonium
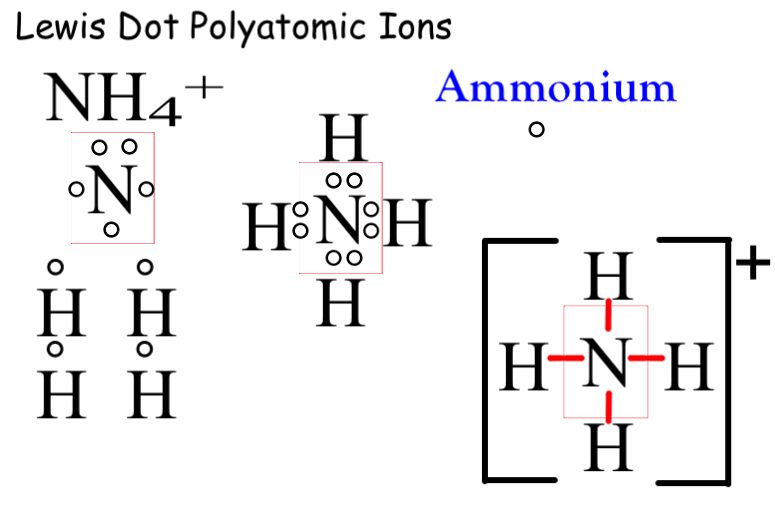
Ammonium Ion Lewis Dot Structure slidesharetrick

NH4+ Lewis Structure Ammonium Ion YouTube
In Order To Draw The Lewis Structure Of Nh4+ Ion, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Nh4+ Ion.
So We've Lost One, That's Minus One.
They Follow The Duet Rule (2 Electrons).
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Related Post: