Draw The Lewis Structure For Co
Draw The Lewis Structure For Co - To determine the total number of valence electrons in co, you need to add up the valence electrons of each atom in the molecule. We have 4 valence electrons for carbon and 6 for oxygen, for a total of 10 valence electrons. Web how to draw the lewis dot structure for carbon monoxide. After determining how many valence electrons there are in co, place them around the central atom to complete the octets. The molecule has one carbon atom and one oxygen atom. This is the co lewis structure: The lewis structure for co has 10 valence electrons. Here are the steps that i follow when drawing a lewis structure. How to draw lewis structure of co 2. In this case, we can condense the last few steps, since not all of them apply. Co formed by adding carbon and oxygen. Calculate the total number of valence electrons. To determine the total number of valence electrons in co, you need to add up the valence electrons of each atom in the molecule. Place the remaining electrons on the atoms; Oxygen is present in group 16. In this case, we can condense the last few steps, since not all of them apply. Calculate the total number of valence electrons. For the co structure use the periodic table to find the total number of valence electrons for. That will normally be the least electronegative atom (c). Put least electronegative atom in centre3. Determine the total number of valence electrons; Here are the steps that i follow when drawing a lewis structure. (valence electrons are the number of electrons present in the outermost shell of an atom). Breaking the octet rule ; Determine the total number of valence electrons in co. Web this video teach you step by step how to draw the lewis structure for co carbon monoxide. For the co lewis structure, calculate the total number of valence electrons for the co molecule. Drawing the lewis structure for co. It is also called chalcogens. The electronic configuration of both ,o = 1s2 2s2 2p4 (6 valence electrons) c =. Co formed by adding carbon and oxygen. That will normally be the least electronegative atom (c). Web this video teach you step by step how to draw the lewis structure for co carbon monoxide. We will first find out the valence electrons for both these atoms and then add both these valence electrons to get the total valence electrons of. Calculate the number of valence electrons: Here are the steps that i follow when drawing a lewis structure. 174k views 3 years ago. Put one electron pair in each bond4. Web this widget gets the lewis structure of chemical compounds. In this case, we can condense the last few steps, since not all of them apply. To determine the total number of valence electrons in co, you need to add up the valence electrons of each atom in the molecule. 174k views 3 years ago. Determine the total number of valence electrons in the molecule or ion. Put one electron. For the co lewis structure, calculate the total number of valence electrons for the co molecule. The electronic configuration of both ,o = 1s2 2s2 2p4 (6 valence electrons) c = 1s2 2s2 2p2 (4 valence electrons). Web there are guidelines (several steps) to draw a lewis structure. Calculate the number of valence electrons: It is 10 to form the. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Oxygen is present in group 16. This is the co lewis structure: Carbon (c) has 4 valence electrons, and oxygen (o) has 6 valence electrons. Web this video teach you step by step how to draw the lewis structure for co carbon. 8 + (2 × × 7) = 22 xef 6: The molecule has one carbon atom and one oxygen atom. Web this video teach you step by step how to draw the lewis structure for co carbon monoxide. It is 6 for one carbon monoxide (co) molecule as per the octet rule. So we have a carbon and an oxygen. 249k views 10 years ago. Drawing the lewis structure for co. Oxygen is present in group 16. How to draw lewis structure for co 2. 8 + (2 × × 7) = 22 xef 6: After determining how many valence electrons there are in co, place them around the central atom to complete the octets. Molecular geometry of co 2. The skeletal structure of co is written as: Web how to draw the lewis dot structure for carbon monoxide. Find how many electrons are needed: So we have a carbon and an oxygen atom bonded together. How to draw lewis structure of co 2. In this case, we can condense the last few steps, since not all of them apply. The electronic configuration of both ,o = 1s2 2s2 2p4 (6 valence electrons) c = 1s2 2s2 2p2 (4 valence electrons). Here, the given molecule is co (carbon monoxide). Count the total number of valence electrons of carbon and oxygen atom.
Steps For Drawing A Lewis Structure Co2 Carbon Dioxide Lewis

How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry
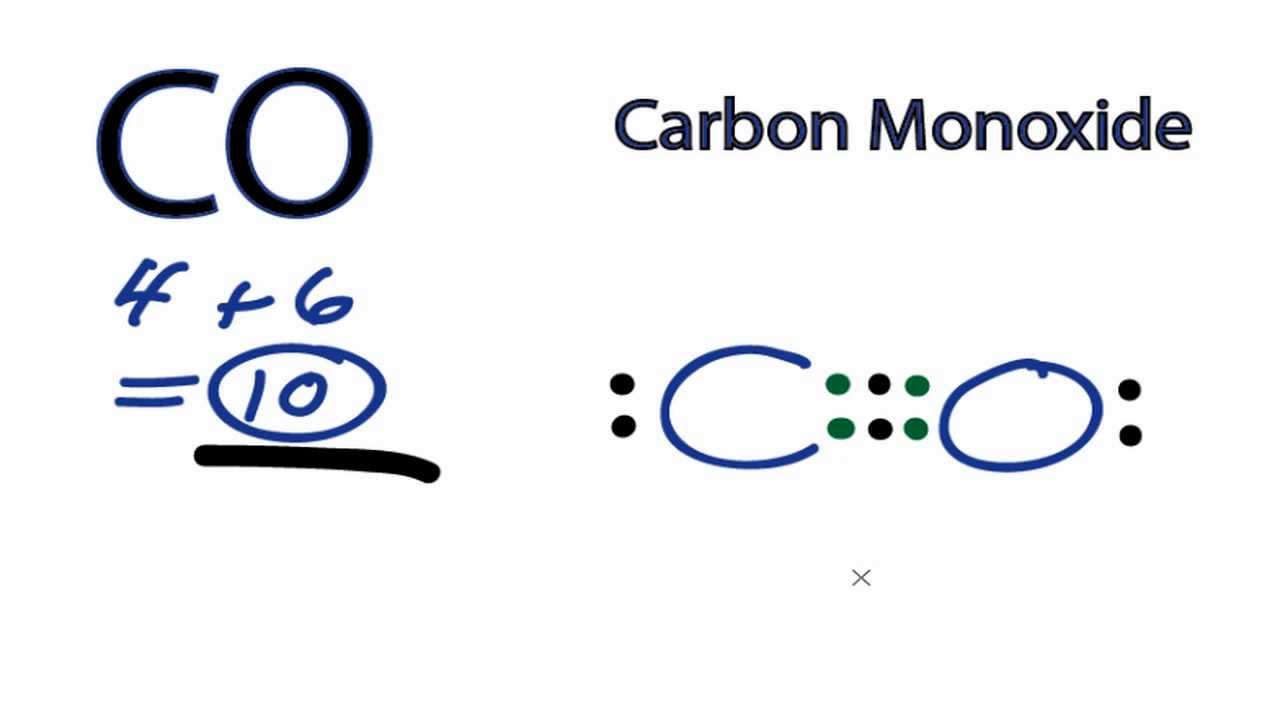
Carbon Monoxide Lewis Structure

Lewis Dot Structure Definition, Examples, and Drawing

How to Draw the Lewis Dot Structure for Carbon monoxide YouTube

How to Draw a Lewis Structure

Carbon Dioxide Lewis Structure How to Draw the Lewis Structure for

Lewis Structure of CO (Carbon Monoxide) YouTube

How do you draw the Lewis structure for CO (Carbon monoxide)?Write
[Solved] 8. Draw the Lewis structures for CO2 and CO, and predict the
The Valence Electrons Available Are 4+6 =10.
Breaking The Octet Rule ;
Determine The Total Number Of Valence Electrons;
Put Least Electronegative Atom In Centre3.
Related Post: