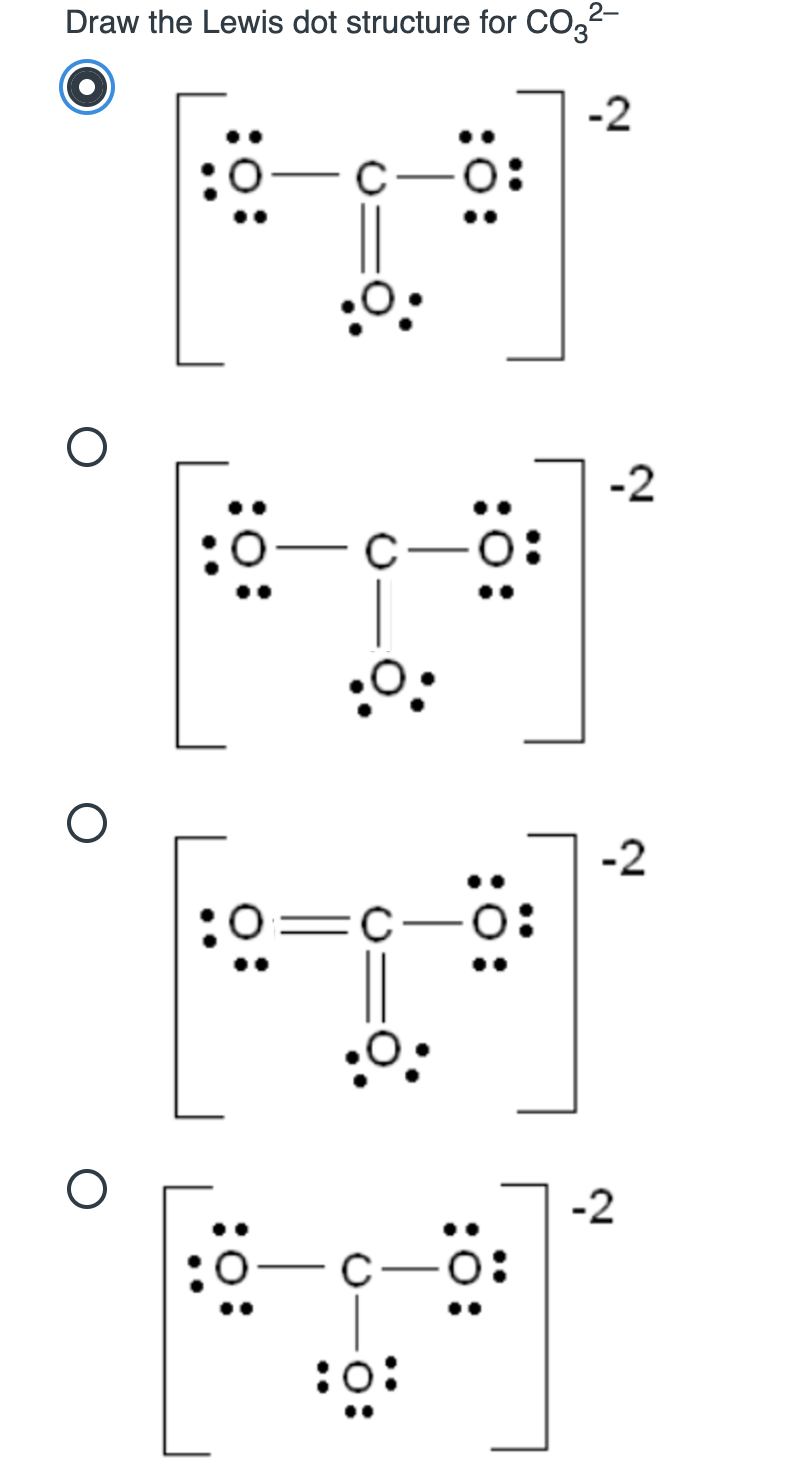
Draw A Lewis Structure For Co32
Draw A Lewis Structure For Co32 - I also go over the resonance, hybridization, shape and bond angle. (the skeleton is indicated by the way the molecule is written.) (a) cl2co (b) h3c—cn (c) h2c—ch2. Web the following lewis diagram represents the valence electron configuration. Web see the big list of lewis structures. Which of the following statements is true?, give the number of valence. (valence electrons are the number of electrons present in the. The remaining electrons are distributed as lone pairs on the oxygen atoms. The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Lewis structure of carbonate ion is drawn in this tutorial step by step. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. In this case, carbon (c) contributes 4 valence electrons, and each oxygen (o) contributes 6 valence electrons, giving a total of 24 valence. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. You will learn. = 4 + 6*3 + 2. The negative charge on the ion is located on two of the oxygen atoms, to indicate. Oxygen has six, we have 3 oxygens, and this negative 2 means we have an extra two valence electrons. Lewis structure of carbonate ion is drawn in this tutorial step by step. You will learn about these facts. In this case, carbon (c) contributes 4 valence electrons, and each oxygen (o) contributes 6 valence electrons, giving a total of 24 valence. (valence electrons are the number of electrons present in the. Determine the central atom of the molecule. Icl in each case, the atom listed first is the central atom. Placing a bonding pair of electrons between each. Single bonds and 1c =o double. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web draw lewis structures for the following species. Carbon is located in group 14 of the periodic table and has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. Calculate the. Write lewis structures that obey the octet rule for each of the following molecules. Describe one resonance structure of the carbonate ion. Connect the atoms to each other with single bonds to form a “skeleton structure.”. (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs (sp). Here’s the. Oxygen has six, we have 3 oxygens, and this negative 2 means we have an extra two valence electrons. Web here’s the best way to solve it. The number of unshared pairs (lone pairs) on the central catom is: Carbon is located in group 14 of the periodic table and has four valence electrons, while oxygen, belonging to group 16,. The lewis structure for co2− 3 is shown in the figure. In carbonate ion, among the two elements, carbon has an. Calculate the total number of valence electrons. Now, in order to draw the lewis structure, we have to determine which one is the central atom in a multiatomic heterogeneous molecule, here an ion. Each of the singly bonded oxygen. Web here’s the best way to solve it. The lewis structure for co2− 3 is shown in the figure. You will learn about these facts in this tutorial. Single bonds and 1c =o double. Write lewis structures that obey the octet rule for each of the following molecules. The negative charge on the ion is located on two of the oxygen atoms, to indicate. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Carbon is the least electronegative, put that at. (valence electrons are the number of electrons present in the. The number of unshared pairs (lone pairs) on the. I also go over the resonance, hybridization, shape and bond angle. Which of the following statements is true? Obeys the octet rule b. Determine the central atom of the molecule. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2). Here’s the best way to solve it. Web see the big list of lewis structures. Which of the following statements is true?, give the number of valence. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: You will learn about these facts in this tutorial. Describe one resonance structure of the carbonate ion. In the lewis structure, the outermost orbit electrons of each atom is shown. Now, in order to draw the lewis structure, we have to determine which one is the central atom in a multiatomic heterogeneous molecule, here an ion. Calculate the total number of valence electrons. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Web draw the lewis structure of the carbonate ion, co 32−. Here’s the best way to solve it. The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs (sp). The number of unshared pairs (lone pairs) on the central catom is: This can be calculated by multiplying the valence electrons of each atom.
Draw the Lewis structure for CO3^2
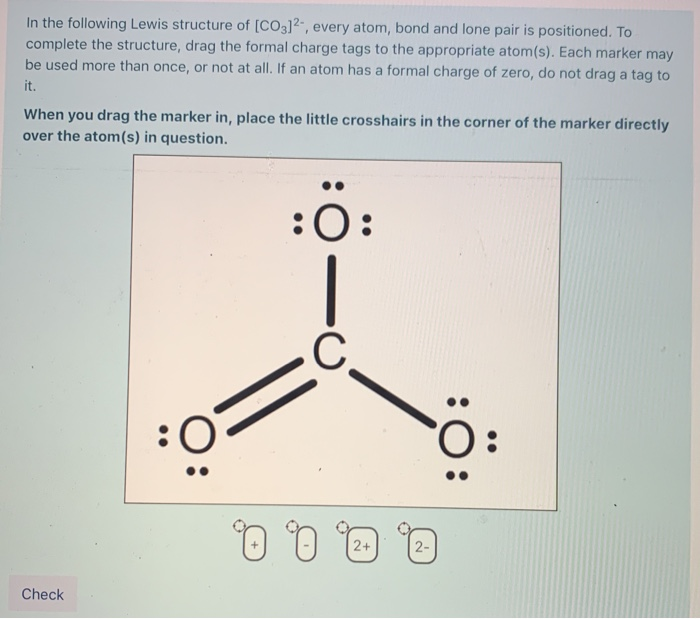
Solved In the following Lewis structure of [CO3)2, every
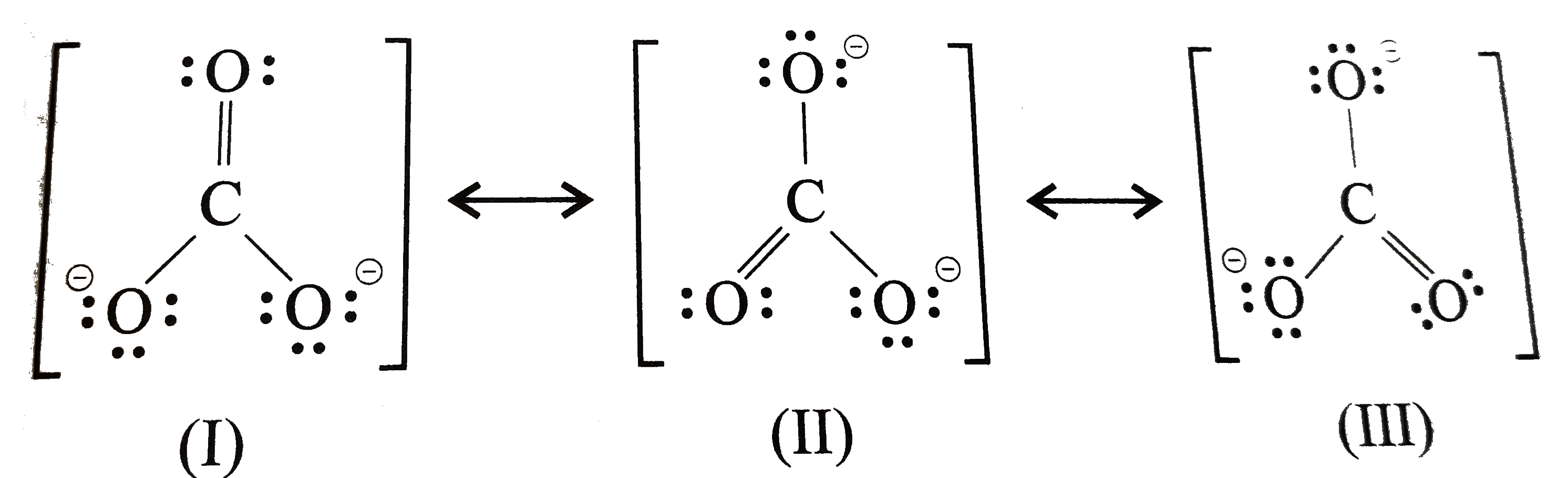
Explain the structure of CO(3)^(2) ion in terms of resonance

How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube

CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2

Draw the Lewis Structure for Co32

CO32 Molecular Geometry, Shape and Bond Angles (Carbonate Ion) YouTube
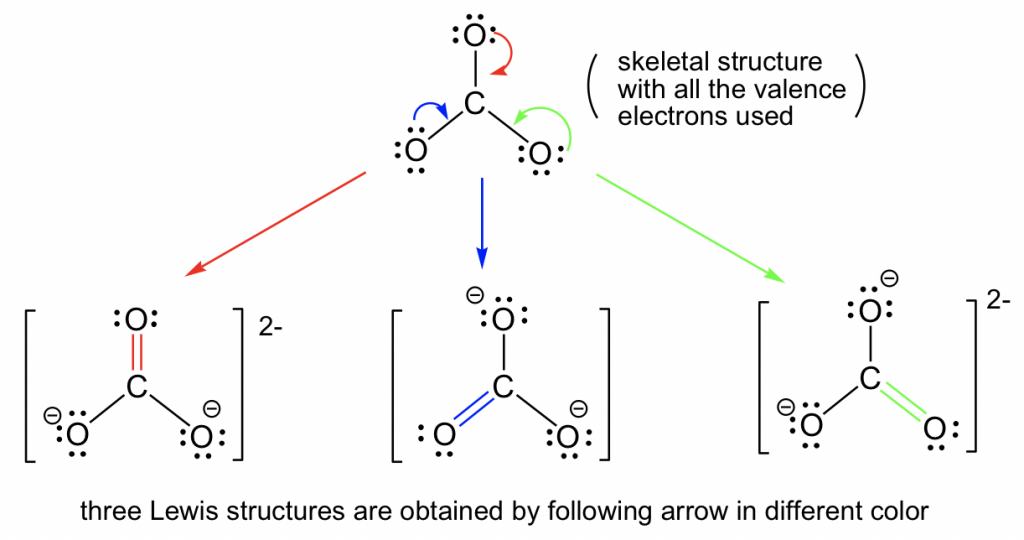
1.3 Resonance Structures Chemistry LibreTexts

CO32 Lewis Structure, Characteristics 13 Facts You Should Know
Solved Draw the Lewis dot structure for CO32 2 0 O 2 0
= 4 + 6*3 + 2.
Obeys The Octet Rule B.
Which Of The Following Statements Is True?
Write Lewis Structures That Obey The Octet Rule For Each Of The Following Molecules.
Related Post: