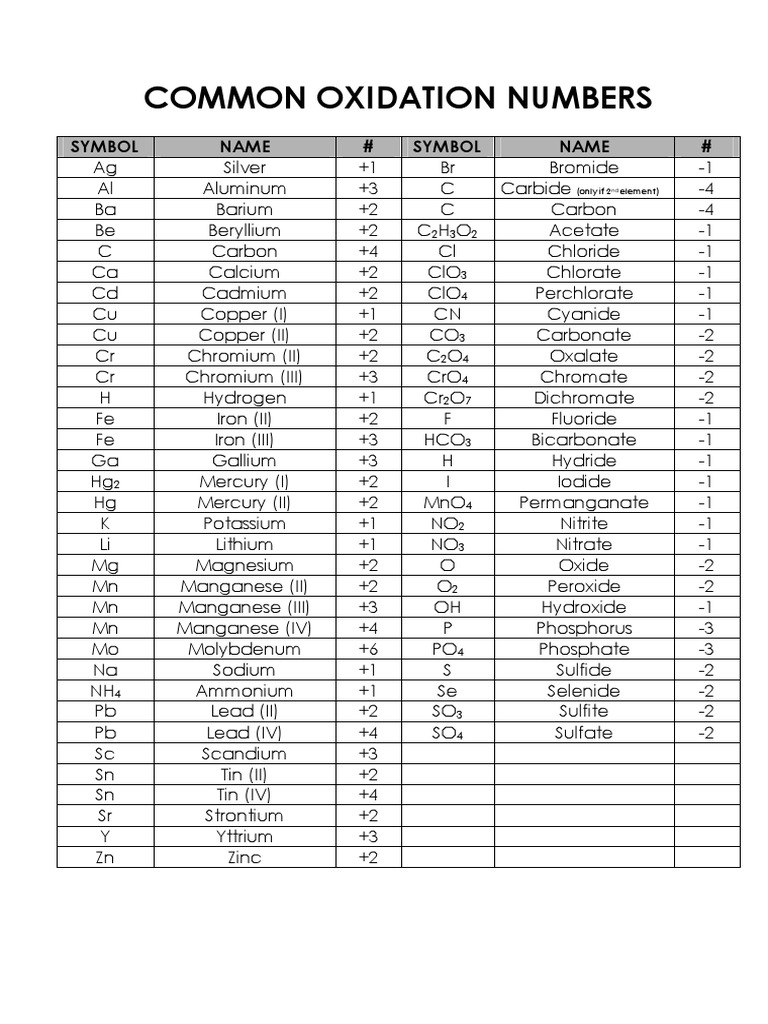
Chart Of Oxidation Number
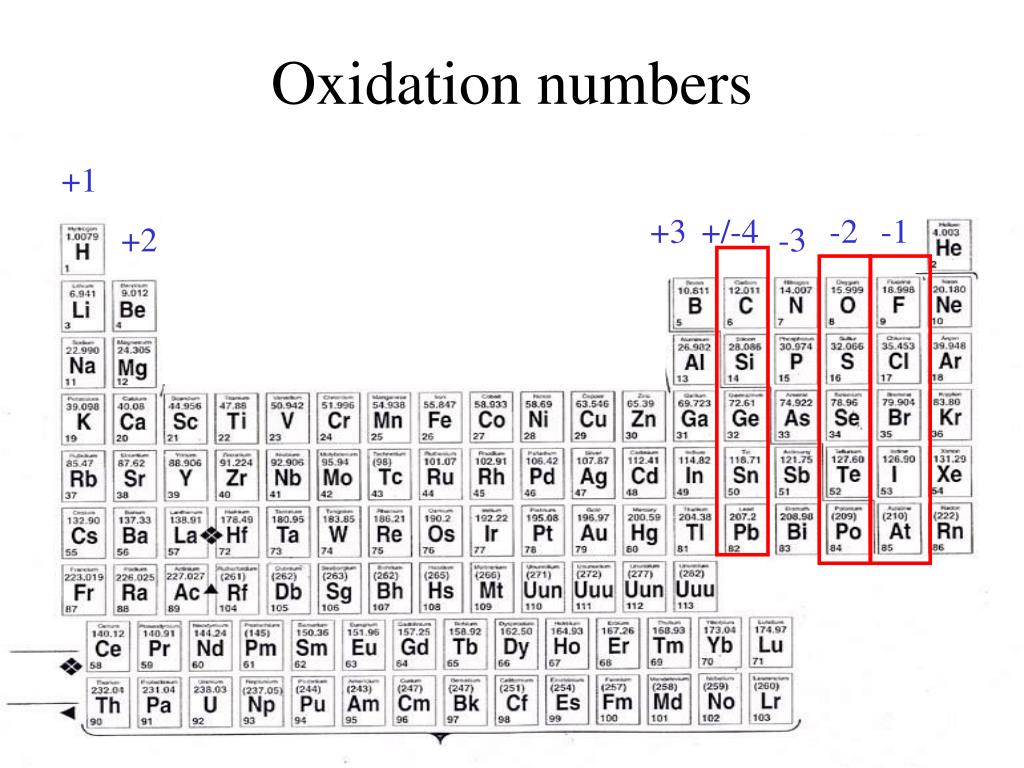
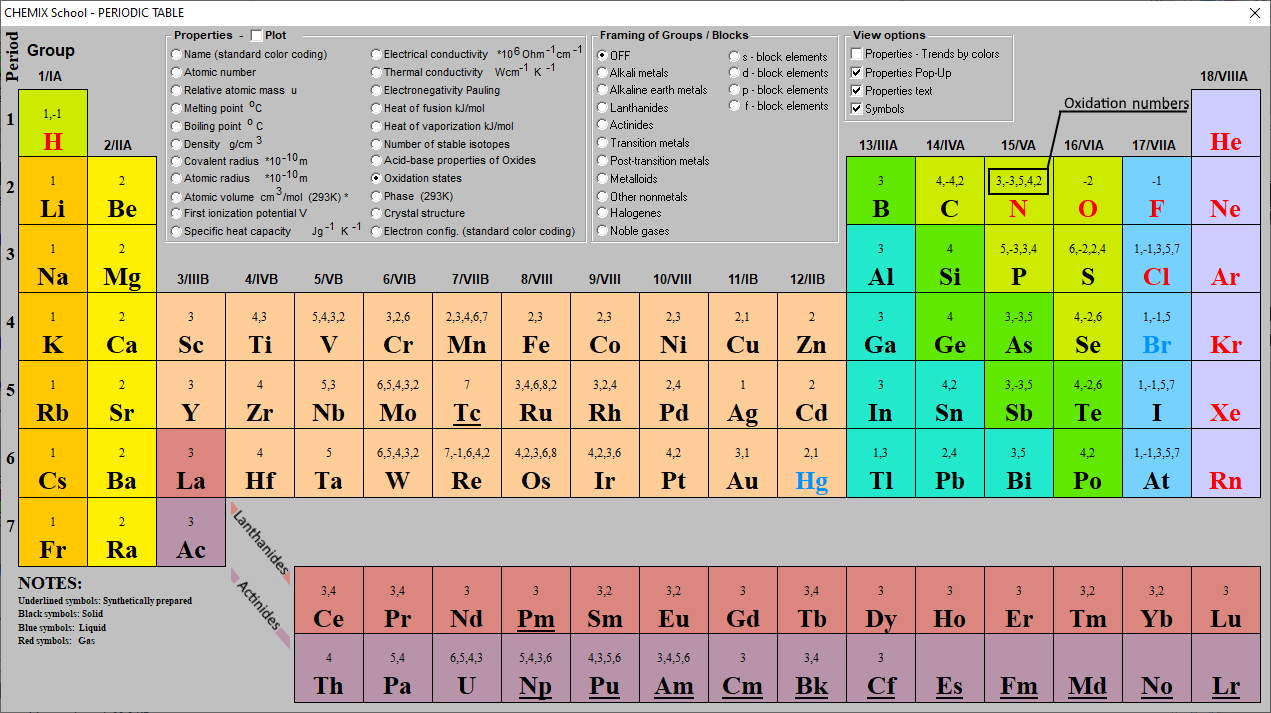
Chart Of Oxidation Number - The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Web the chart below should help you to visualize the possible oxidation numbers that can occur for the first 39 atoms. Web in simple words, the oxidation number is the number assigned to the components in a chemical combination. Web the following table lists the preferred (in bold), occasionally occurring, observed under certain conditions (in parentheses) and theoretically predicted oxidation states (in brackets) of the various chemical elements; The oxidation number of hydrogen is +1 when it is combined with a nonmetal. Web showing 1 to 59 of 59 entries. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Information of oxidation numbers of monoatomic ions in periodic table. You can download the chart and the table above by clicking on either. Web for example, iron common has an oxidation number of +2 or +3. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Web the chart below should help you to visualize the possible oxidation numbers that can occur for the first 39 atoms. Oxidation states of s block. Each atom in a redox reaction is assigned an oxidation number to understand. We can use oxidation numbers to keep track of where electrons are in a molecule, and how they move during a reaction. In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in a compound. This is a list of all the known oxidation states of the chemical. Web the following table lists the preferred (in bold), occasionally occurring, observed under certain conditions (in parentheses) and theoretically predicted oxidation states (in brackets) of the various chemical elements; You can't actually do that with vanadium, but you can with an element like sulphur. To keep track of electrons in a. As the table shows, the presence of the other. Redox reactions are characterized by a transfer of electrons. The most common oxidation states are in bold. Web the following table lists the preferred (in bold), occasionally occurring, observed under certain conditions (in parentheses) and theoretically predicted oxidation states (in brackets) of the various chemical elements; Web this printable periodic table contains the number, symbol, name, atomic mass and oxidation. This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. If you're working out the oxidation states of the atoms in a reaction and you get one that's not on this chart, it's probably worth checking your work. In addition, the electron configurations of neutral atoms (oxidation state zero; Web charges given to. Remember, shells don’t neatly stack on top of each other, so valence (and oxidation state) may not be the same as the total number of electrons in the outer shell. Zn + 2h+ zn2+ +h2 zn + 2 h + zn 2 + + h 2. Web showing 1 to 59 of 59 entries. Web charges given to atoms in. If you're working out the oxidation states of the atoms in a reaction and you get one that's not on this chart, it's probably worth checking your work. Organic compounds and some covalent compounds do not have oxidation states assigned to the atoms in the. Web this printable periodic table contains the number, symbol, name, atomic mass and oxidation states. Information of oxidation numbers of monoatomic ions in periodic table. To keep track of electrons in a. Lih, nah, and cah 2. Web the chart below should help you to visualize the possible oxidation numbers that can occur for the first 39 atoms. In addition, the electron configurations of neutral atoms (oxidation state zero; Each atom in a redox reaction is assigned an oxidation number to understand its ability to donate, accept, or share electrons. First previous 1 next last. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Remember, shells don’t neatly stack on top of each other, so valence (and oxidation state) may not. Redox reactions are characterized by a transfer of electrons. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. To keep track of electrons in a. Lih, nah, and cah 2. This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Each atom in a redox reaction is assigned an oxidation number to understand its ability to donate, accept, or share electrons. Web what is the oxidation number when an element has not combined or do not form a compound. Web the oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction. Web what if you kept on adding electrons to the element? A net ionic charge can be specified at the end of the compound between { and }. Remember, shells don’t neatly stack on top of each other, so valence (and oxidation state) may not be the same as the total number of electrons in the outer shell. The oxidation number of hydrogen is +1 when it is combined with a nonmetal. Web this printable periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. To keep track of electrons in a. The oxidation number is basically the count of electrons that atoms in a molecule can share, lose or gain while forming chemical bonds with other atoms of a different element. The oxidation number, in simple terms, can be described as the number that is allocated to elements in a chemical combination. Organic compounds and some covalent compounds do not have oxidation states assigned to the atoms in the. Web the following table lists the preferred (in bold), occasionally occurring, observed under certain conditions (in parentheses) and theoretically predicted oxidation states (in brackets) of the various chemical elements; This table is available for download as a pdf file and printed for offline use. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion.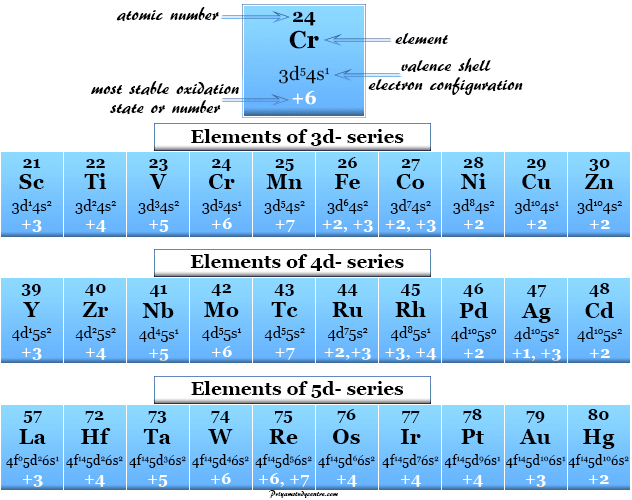
Oxidation Number Periodic table elements Definition, Rules (2023)
Periodic Table Oxidation Chart

Downloadable Periodic Table Oxidation States
Oxidation Numbers Periodic Table Elements
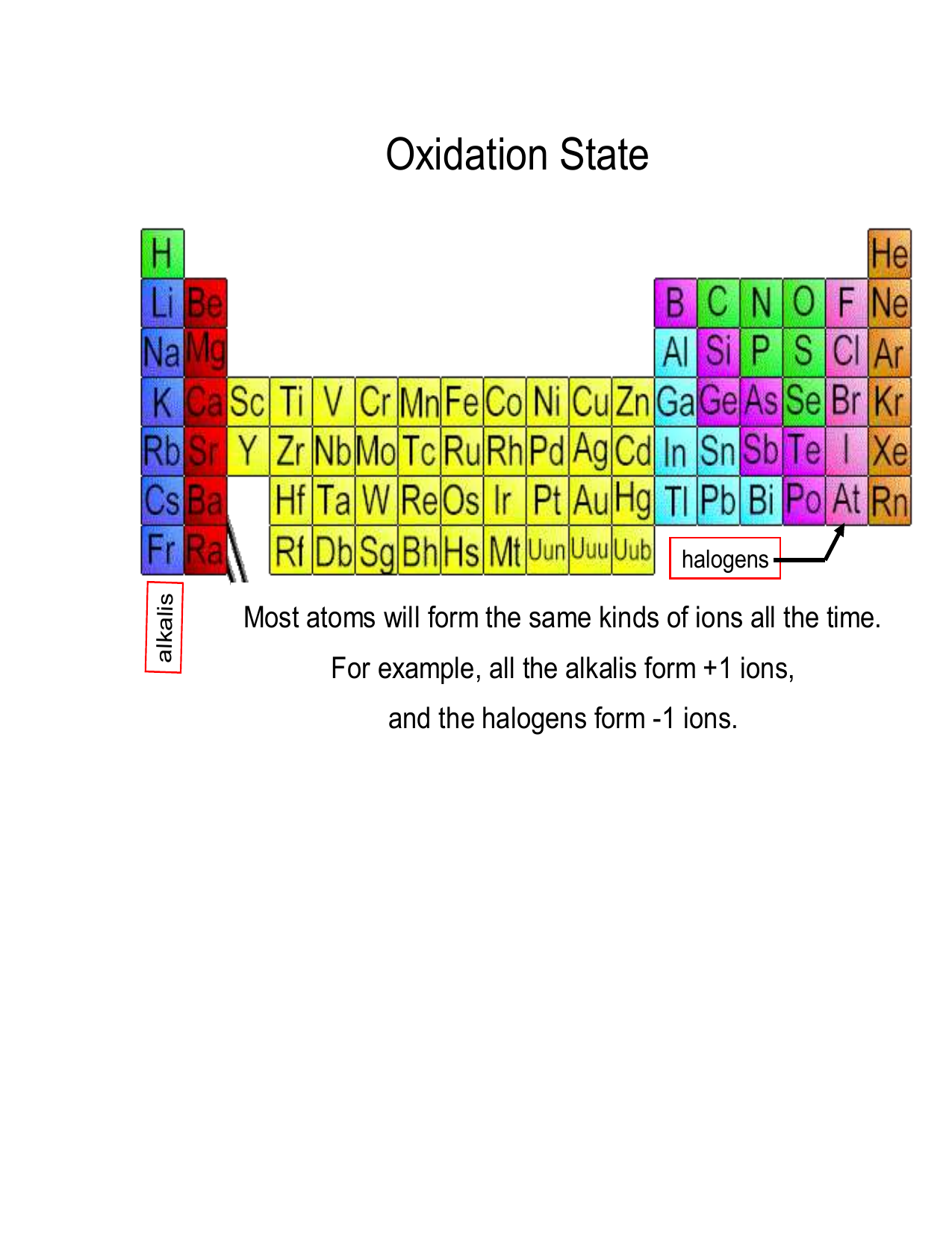
Oxidation Numbers
Common Oxidation Numbers Chart

oxidation states Archives Science Notes and Projects

Oxidation Number (State) Definition, Rules, How to Find, and Examples

Labeled Periodic Table With Oxidation Numbers Periodic Table Timeline
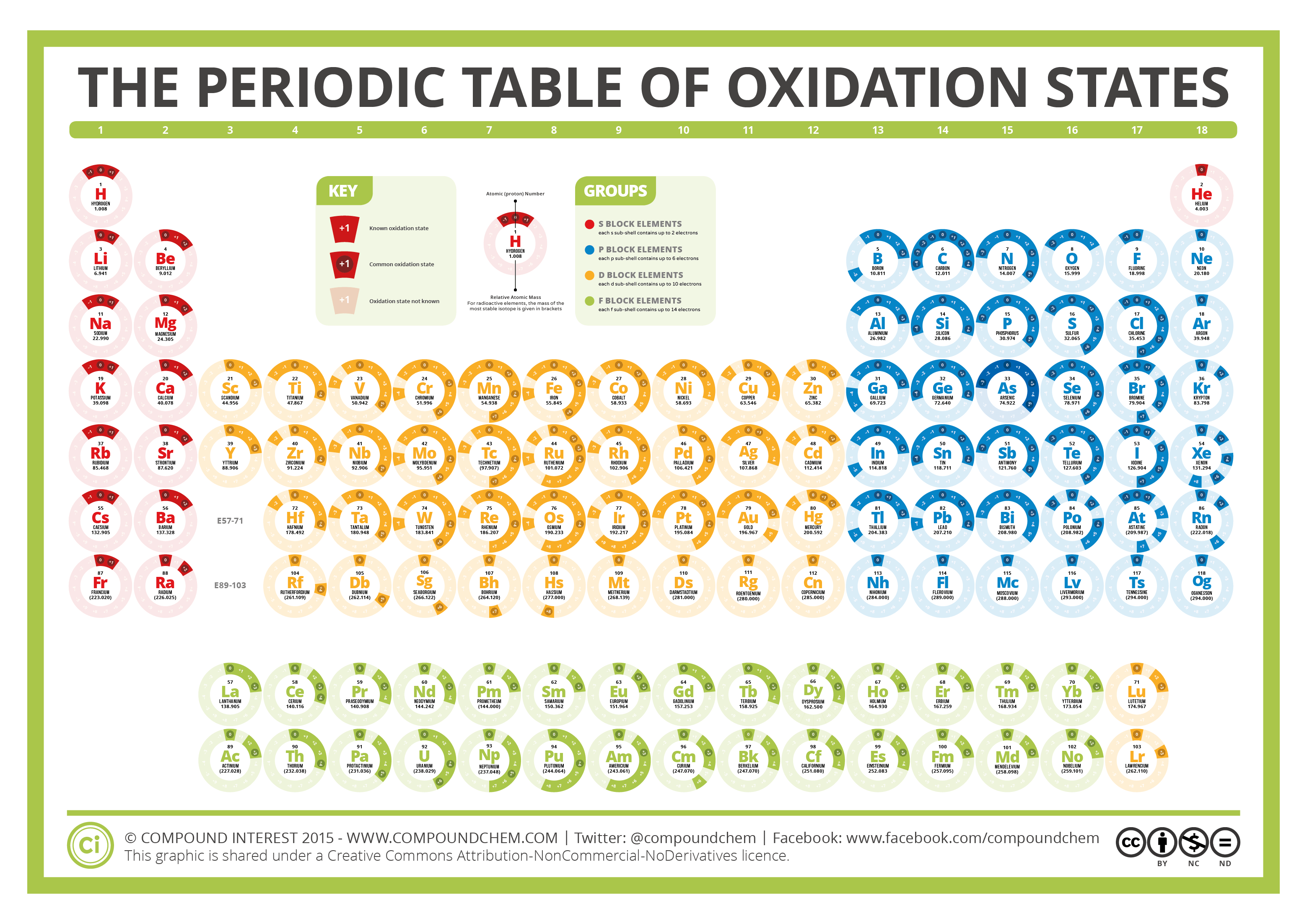
The Periodic Table of Oxidation States Compound Interest
Oxidation Number Of An Atom When An Element Has Combined With The Same Element.
You Can Download The Chart And The Table Above By Clicking On Either.
Web This Color Periodic Table Contains The Number, Symbol, Name, Atomic Mass And Oxidation States Of Each Element.
If You're Working Out The Oxidation States Of The Atoms In A Reaction And You Get One That's Not On This Chart, It's Probably Worth Checking Your Work.
Related Post: