Ch4 Drawing
Ch4 Drawing - Determine the total number of valence electrons in the molecule. Place the carbon atom in the center and connect it with four hydrogen atoms using single bonds. In order to draw the lewis structure of ch4, first of all you have to find the total number of valence electrons present in the ch4 molecule. Tetracyanomethane or carbon tetracyanide is a percyanoalkane molecular carbon nitride with formula c(cn)₄. There are no lone pairs in the valence shells of carbon atom. The molecular formula tells the symbols of the elements that compose the compound, and the subscript to the element symbol denotes how many atoms of that element are in the molecule. Generally, the lone pairs in the molecule distort the shape of the molecule, which changes the molecule’s bond angles. Web the ch4 molecule will have 109.5° bond angles as there is no distortion in its shape. It is a colourless, flammable gas. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary. Web i quickly take you through how to draw the lewis structure of methane, ch4. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of ch4 is sp3. In the ch4 lewis structure, the molecule is a tetrahedral shape and has a bond angle of. The modern structure shows that there are only 2 unpaired electrons to share. In the ch4 lewis structure, the molecule is a tetrahedral shape and has a bond angle of perfectly 109.50. Web drawing the lewis structure for ch 4. Tetracyanomethane or carbon tetracyanide is a percyanoalkane molecular carbon nitride with formula c(cn)₄. Web drawing the ch4 lewis structure. Web the lewis structure of the methane (ch4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Explore molecule shapes by building molecules in 3d! In order to draw the lewis structure of ch4, first of all you. Web in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. Drawing the ch4 lewis structure is a simple and straightforward process that can be done in a few easy steps. Here’s how to do it: It. How does molecule shape change with different numbers of bonds and electron pairs? Web pentane is a compound that contains four tetrahedral carbons in a row. Web check me out: There are following specifications in the lewis structure of methane. Web we recommend using the latest version of chrome, firefox, safari, or edge. Calculate the total number of valence electrons. Place the carbon atom in the center and connect it with four hydrogen atoms using single bonds. There are following specifications in the lewis structure of methane. It is a colourless, flammable gas. It is interesting to realize that irrespective. Methane is produced naturally from rotting vegetation in marshes. It is interesting to realize that irrespective. One h atom is below the molecular plane and the other is above the. Web check me out: Explore molecule shapes by building molecules in 3d! Molview consists of two main parts, a structural formula editor and a 3d model viewer. Place the carbon atom in the center and connect it with four hydrogen atoms using single bonds. For ch 4 you have a total of 8 total valence electrons. It is the simplest hydrocarbon in the organic molecule and it is a hydride of c.. Drawing the ch4 lewis structure is a simple and straightforward process that can be done in a few easy steps. Web check me out: Here’s how to do it: How does molecule shape change with different numbers of bonds and electron pairs? Draw the lewis structure of methane (ch4). The modern structure shows that there are only 2 unpaired electrons to share. Drawing the ch4 lewis structure is a simple and straightforward process that can be done in a few easy steps. Web 6 steps to draw the lewis structure of ch4 step #1: Web in order to understand the hybridization of ch 4 (methane), we have to take. Carbon (c) = 4 valence electrons hydrogen (h) = 1 valence electron x 4 = 4 valence electrons total = 4 + 4 = 8 valence electrons. In order to draw the lewis structure of ch4, first of all you have to find the total number of valence electrons present in the ch4 molecule. The type of hybridization involved with ch4 is sp 3. In the ch 4 lewis structure, there are four single bonds around the carbon atom,. Web drawing the ch4 lewis structure. The molecular formula tells the symbols of the elements that compose the compound, and the subscript to the element symbol denotes how many atoms of that element are in the molecule. Web in order to understand the hybridization of ch 4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. Drawing the lewis structure for ch 4 (named methane) requires only single bonds.it's one of the easier lewis structures to draw. Carbon atom is the center atom and it is very easy to draw ch4 lewis structure. This is because they can share a maximum of two electrons. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary. There are following specifications in the lewis structure of methane. Draw the lewis structure of methane (ch4). Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Here’s how to do it: Explore molecule shapes by building molecules in 3d!
Electron Dot Diagram For Methane

CH4 Lewis Structure, Molecular Geometry, and Hybridization
Methane Molecule CH4 Drawing Drawing by Frank Ramspott Fine Art America
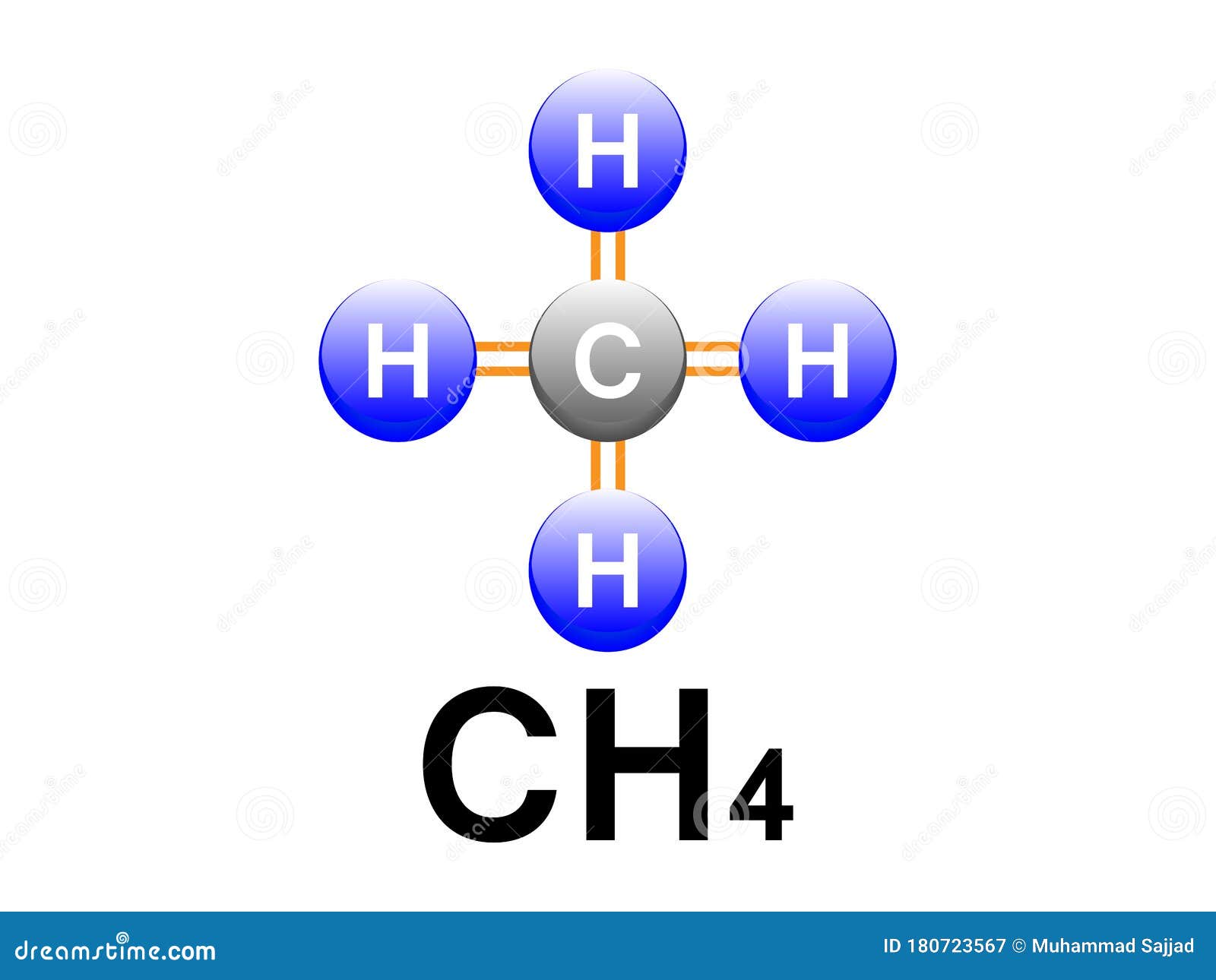
CH4 Methane Covalent Bonding .Methane Formula Diagram Design for
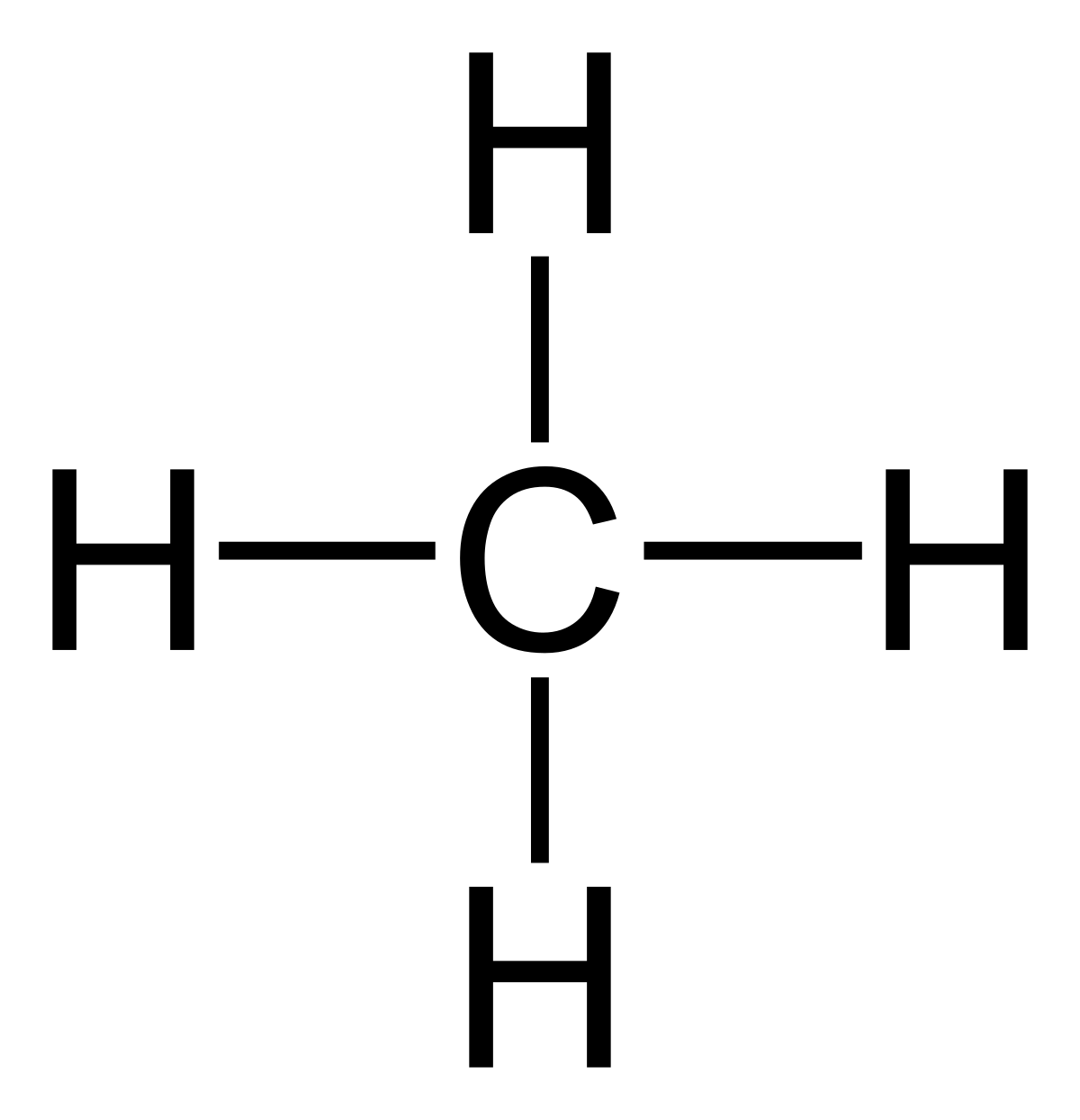
Dot Diagram For Ch4

Ch4 Electron Dot Diagram
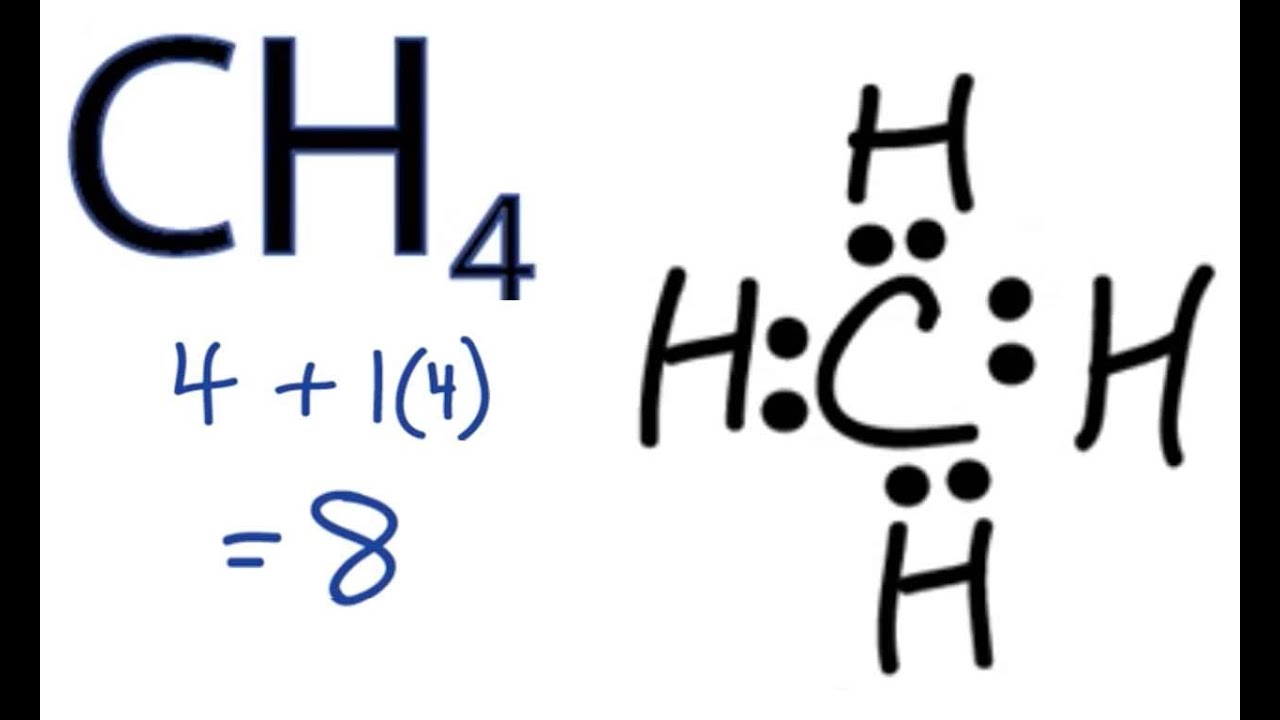
CH4 Lewis Structure How to Draw the Dot Structure for CH4 (Methane

Draw Lewis Structure For Ch4 Nelson Tardwilis

How to Draw the Lewis Dot Structure for CH4 Methane YouTube
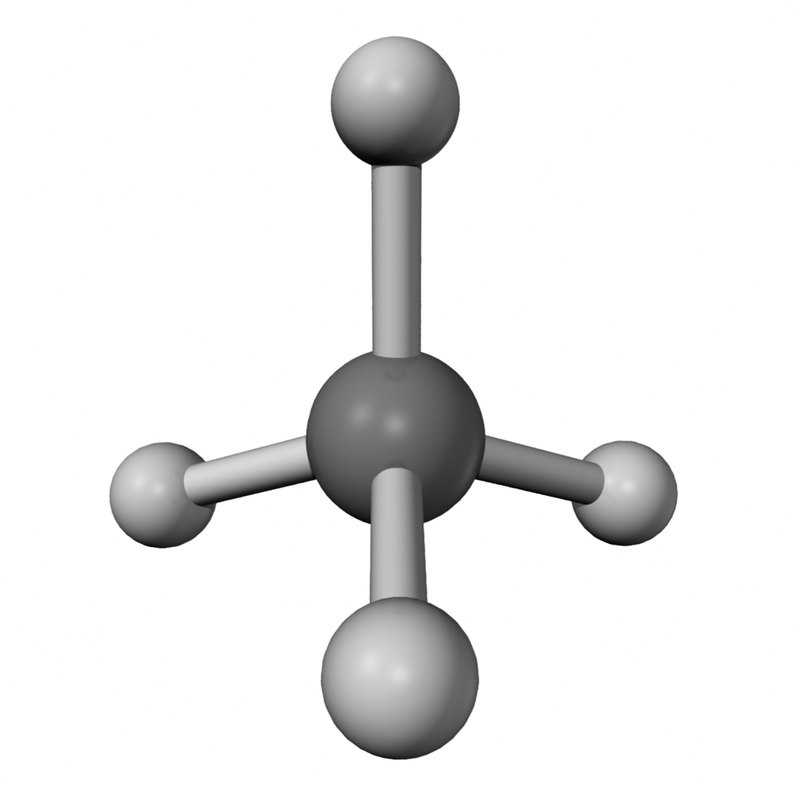
ch4 obj
Web Drawing The Lewis Structure For Ch 4.
Once They Have Two Valence Electrons Their Outer Shell Is.
Web Pentane Is A Compound That Contains Four Tetrahedral Carbons In A Row.
Drawing The Ch4 Lewis Structure Is A Simple And Straightforward Process That Can Be Done In A Few Easy Steps.
Related Post: