Cdrh Organization Chart
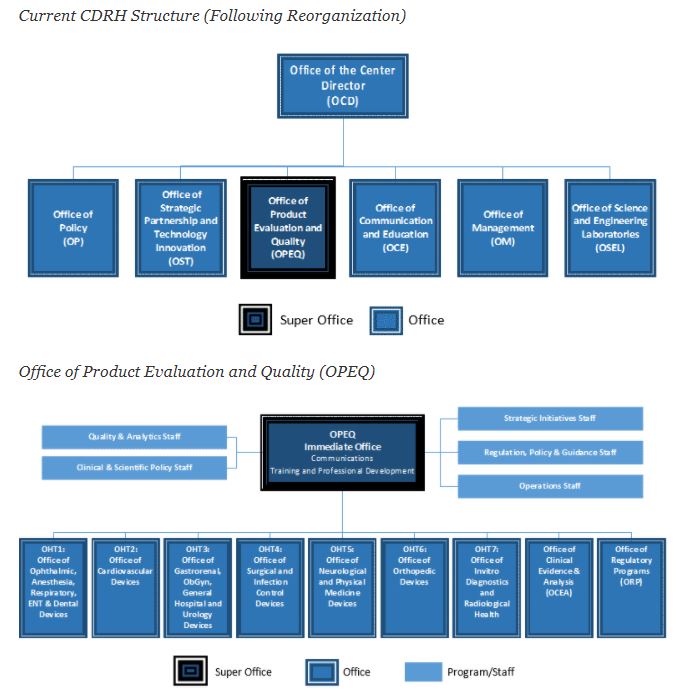
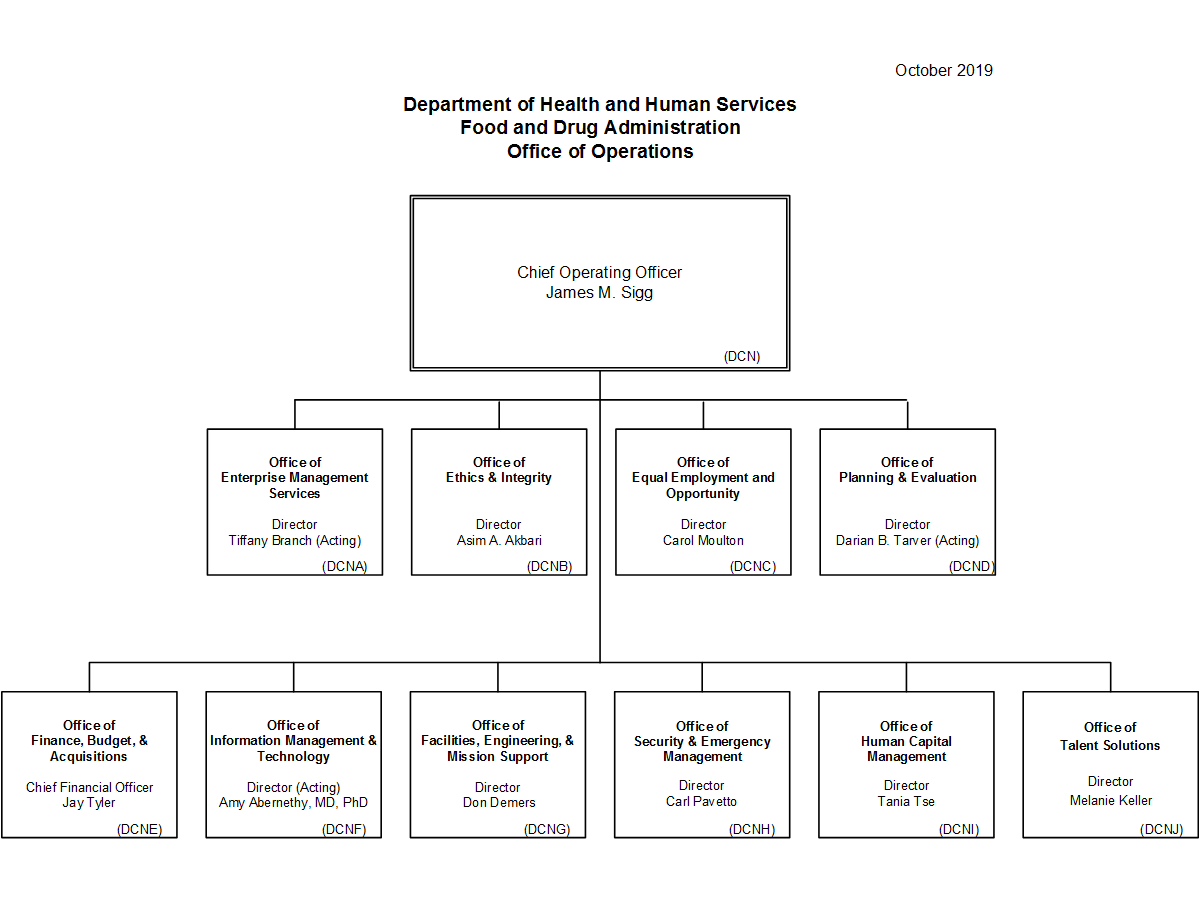
Cdrh Organization Chart - Issued may 2, 2002 the fda's center for devices and radiological health (cdrh) works to ensure public safety by certifying the safety and effectiveness of medical and radiologic devices in. Reorganization of the center for devices and radiological health. This list enables you to view charts of performance data and progress on important projects and programs. Web the current cdrh structure, following reorganization, follows: Although many of the senior staff within these new offices will remain the same, they will now have new titles. Web per the cdrh release, its primary functions include an external focus on issues such as “medical device cybersecurity, digital health, standards, and patient science,” and coordinating the center’s readiness for crisis events such as pandemic outbreak or supply chain shortages. The program contributes to the center’s mission of protecting and promoting public health through the development and recognition of voluntary consensus standards that serve to establish safe and effective. Web content current as of: Organization chart for the fda's center for devices and radiological health, including leadership roles. Web previous cdrh organization model. The cdrh standards program was established as a result of the food and drug administration modernization act (fdama) of 1997. For specific phone numbers and email addresses for each. Cdrh mailing addresses and office phone numbers; Web free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics Organization chart for the fda's center for devices and. The center for devices and radiological health (cdrh) is the branch of the united states food and drug administration (fda) responsible for the premarket approval of all medical devices, as well as overseeing the manufacturing, performance and safety of these devices. Cdrh mailing addresses and office phone numbers; The cdrh standards program was established as a result of the food. The following bullets capture the cdrh management directory, by organization: Web free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics Department of health and human. Cdrh mailing addresses and office phone numbers; 3 old cdrh structure super office office division branch team program/staff office of the center director Reorganization of the center for devices and radiological health. Web • cdrh reorganization includes adopting a tplc model and other efforts to streamline and improve efficiency and to support professional growth • implementation timeline: Web cdrh office of communication and education organization chart food and drug administration office of medical products and tobacco center for devices and radiological health office. Office of center director (ocd) office of compliance (oc) office of device evaluation (ode) office of in vitro diagnostics and radiological health (oir) office of surveillance and biometrics (osb) communication and education (oce) office of management (om) office of science Web content current as of: Department of health and human. Web per the cdrh release, its primary functions include an. Web the current cdrh structure, following reorganization, follows: Reorganization of the center for devices and radiological health. Web • cdrh reorganization includes adopting a tplc model and other efforts to streamline and improve efficiency and to support professional growth • implementation timeline: Web free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics Web the cdrh. Submitting reports and requirements for maintaining records for. Web free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics Web the list below provides information on fda's performance measures and projects as aligned to fda strategic priorities and program offices. Issued may 2, 2002 the fda's center for devices and radiological health (cdrh) works to ensure. The following header reflects the organizational hierarchy. Web keith began by sharing the current cdrh organizational chart, which was finalized in october 2019. The cdrh standards program was established as a result of the food and drug administration modernization act (fdama) of 1997. Web though there are additional changes observed in the organizational chart, those discussed above will likely be. Cdrh management directory by organization; Office of center director (ocd) office of compliance (oc) office of device evaluation (ode) office of in vitro diagnostics and radiological health (oir) office of surveillance and biometrics (osb) communication and education (oce) office of management (om) office of science Cdrh mailing addresses and office phone numbers; Web though there are additional changes observed in. Web cdrh organization contacts. Reorganization of the center for devices and radiological health. The program contributes to the center’s mission of protecting and promoting public health through the development and recognition of voluntary consensus standards that serve to establish safe and effective. Cdrh management directory by organization; Issued may 2, 2002 the fda's center for devices and radiological health (cdrh). The program contributes to the center’s mission of protecting and promoting public health through the development and recognition of voluntary consensus standards that serve to establish safe and effective. Web cdrh organization contacts. Web per the cdrh release, its primary functions include an external focus on issues such as “medical device cybersecurity, digital health, standards, and patient science,” and coordinating the center’s readiness for crisis events such as pandemic outbreak or supply chain shortages. Although many of the senior staff within these new offices will remain the same, they will now have new titles. The following bullets capture the cdrh management directory, by organization: Cdrh management directory by organization; The following header reflects the organizational hierarchy. Reorganization of the center for devices and radiological health. Web the cdrh organizational chart is updated quarterly. Web previous cdrh organization model. Web keith began by sharing the current cdrh organizational chart, which was finalized in october 2019. Cdrh mailing addresses and office phone numbers; Submitting reports and requirements for maintaining records for. Cdrh management directory by organization. Web the current cdrh structure, following reorganization, follows: Office of center director (ocd) office of compliance (oc) office of device evaluation (ode) office of in vitro diagnostics and radiological health (oir) office of surveillance and biometrics (osb) communication and education (oce) office of management (om) office of science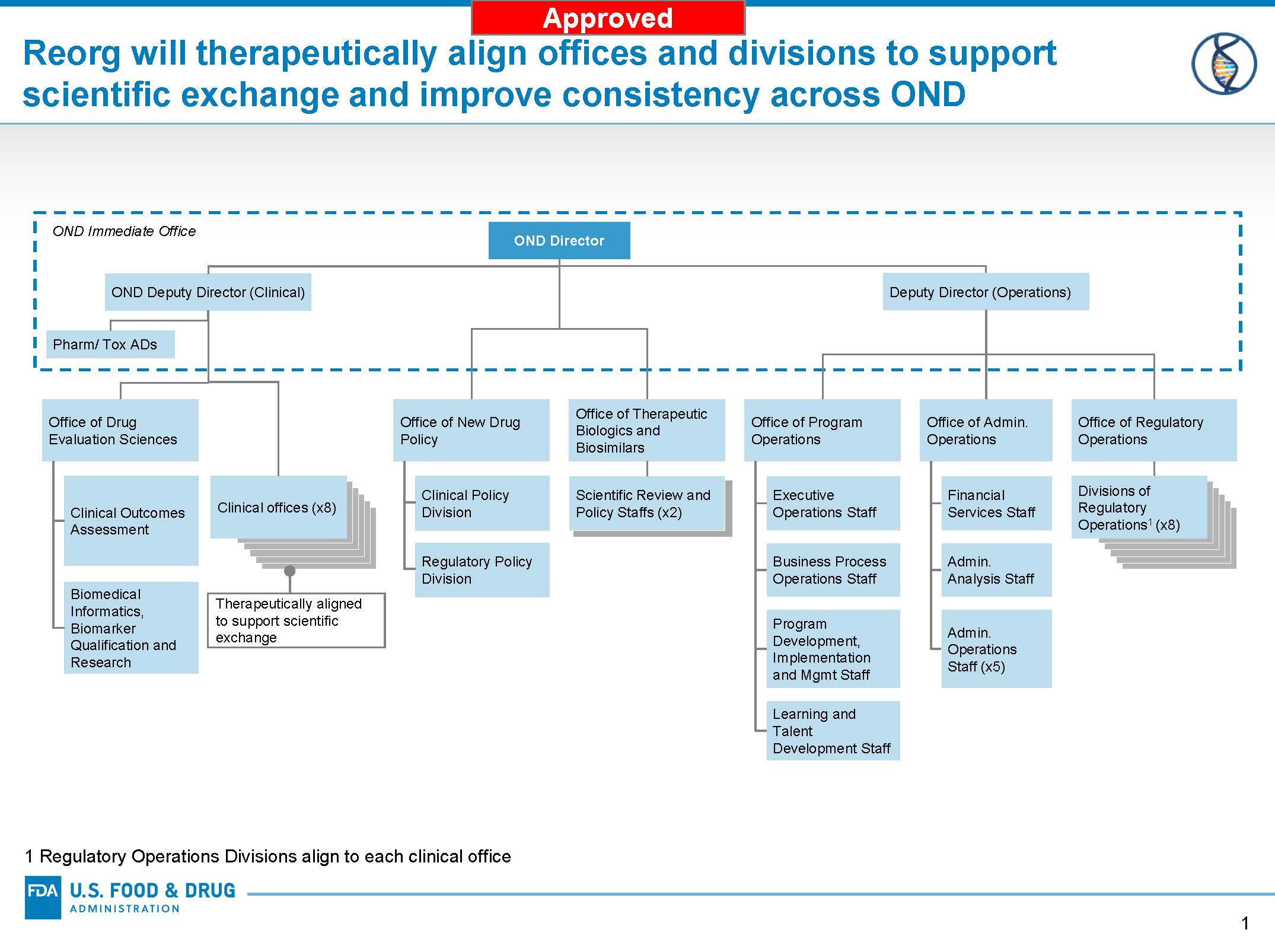
Of The Office Of New Drugs With Corresponding Changes To

PreApplication Information Webinar for PAR21183, "Developing Digital
Center For Device and Radiological Health Dawnbreaker MRR

Organizational Structure of the FDA. Download Scientific Diagram
Operational Organizational Chart
Fillable Org Chart Learn Diagram vrogue.co

Download Template Desain Kemasan Produk Cdrh Organizational Chart
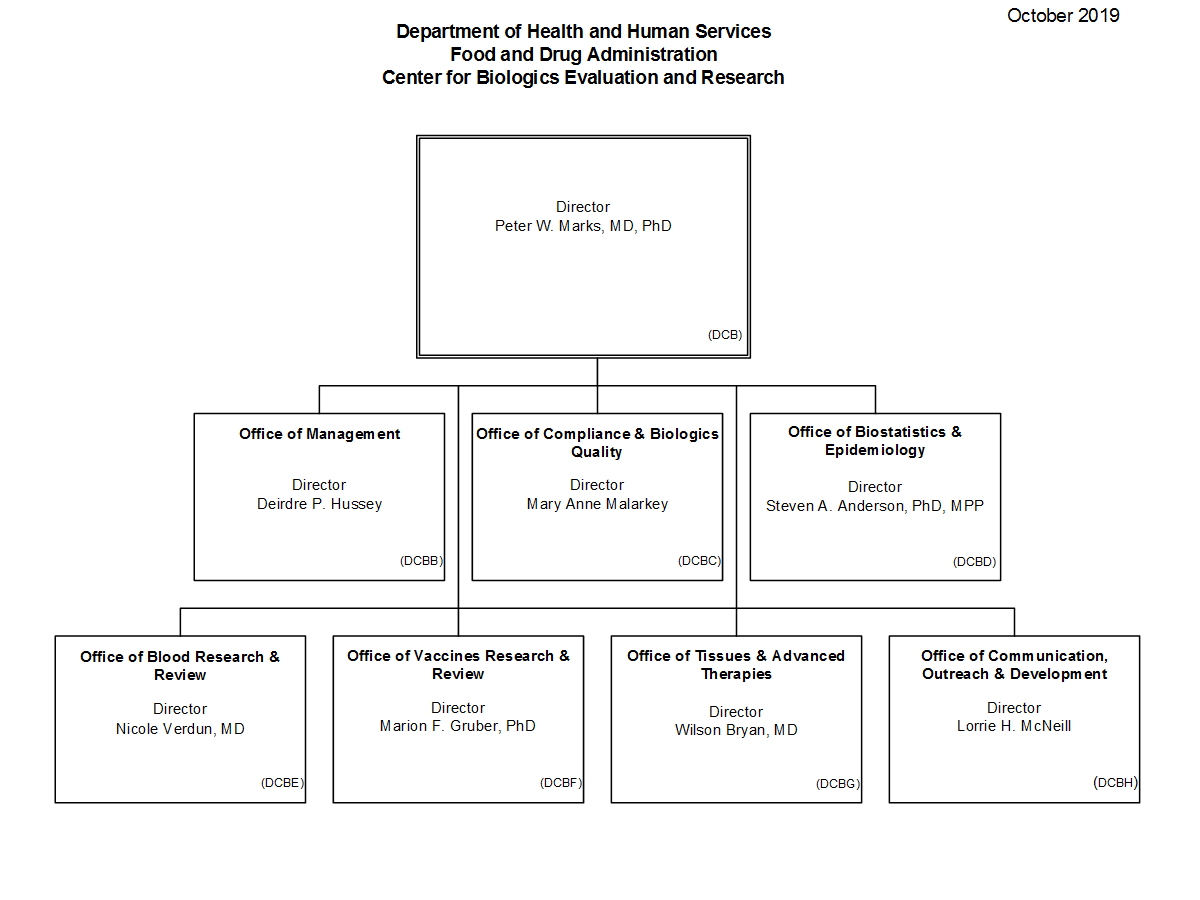
Center for Biologics Evaluation and Research Organization Chart FDA
FDA CDRH Organizational Structure & Overview

It’s Now Official The New CDRH Organizational Structure and How It May
Issued May 2, 2002 The Fda's Center For Devices And Radiological Health (Cdrh) Works To Ensure Public Safety By Certifying The Safety And Effectiveness Of Medical And Radiologic Devices In.
At The Top Of This Structure, You'll Find The Office Of The Center Director.
Department Of Health And Human.
Web Cdrh Office Of Communication And Education Organization Chart Food And Drug Administration Office Of Medical Products And Tobacco Center For Devices And Radiological Health Office Of Communication And Education.
Related Post: