Bond Length Chart
Bond Length Chart - Bond order and length are inversely proportional to each other: Web the bond length between any two adjacent nuclei in such a covalent molecule is the distance between the two nuclei at the minimum energy in a graph of energy versus nuclear separation. I know this is a late response, but from what i gather we can tell what the bond order is by looking at the number of valence electrons and how many electrons the atoms need to share to complete their outer shell. Darwent, national standard reference data series, national bureau of standards, no. For example, the bond length in a h 2 molecule is 74 pm, as shown in this figure. Bond energy is the potential energy required to break a covalent bond. The bond length is internuclear distance of two covalently bonded atoms. Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond). To define and used average bond energies. The larger the bond energy, the stronger the covalent bond is. Bond order is the number of electron pairs that hold two atoms together. Web to calculate bond length, one must draw the lewis structure for the molecule, find the atomic radii of the two atoms on an atomic radius chart, and add the two atomic radii together. Bond length & bond strength. I know this is a late response, but. Darwent, national standard reference data series, national bureau of standards, no. Before we go into the details explaining the bong lengths and bond strengths in organic chemistry, let’s put a small summary for these two properties right from the beginning as it stays relevant for all types of bonds we are going to talk about. Web bond order and bond. Experimental > geometry > diatomic bond lengths list of experimental diatomic bond lengths units are: When bond order is increased, bond length is decreased. Bond length to change units) In proposing his theory that octets can be completed by two atoms sharing electron pairs, lewis provided scientists with the first description of covalent bonding. The bond energy is inversely proportional. The bond energy is the energy required to break one mole of a particular covalent bond in the gaseous states. Lewis diagram of xenon difluoride (xef₂) practice. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Web bond order and bond length indicate the type and strength of. Lewis diagram of formaldehyde (ch₂o) worked example: Cottrell, the strengths of chemical bonds, 2nd ed., butterworths, london, 1958; Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. As we go across a period we see bond length decreases. When bond order is increased, bond length is decreased. The bond energy is the energy required to break one mole of a particular covalent bond in the gaseous states. Bond length is the average distance between the two nuclei of atoms bonded together in a covalent bond. ) and bond lengths (r) reference: The bond length is internuclear distance of two covalently bonded atoms. As we go across a. Web the bond length between any two adjacent nuclei in such a covalent molecule is the distance between the two nuclei at the minimum energy in a graph of energy versus nuclear separation. Lewis diagram of formaldehyde (ch₂o) worked example: A shorter bond length has higher bond energy. Darwent, national standard reference data series, national bureau of standards, no. The. Before we go into the details explaining the bong lengths and bond strengths in organic chemistry, let’s put a small summary for these two properties right from the beginning as it stays relevant for all types of bonds we are going to talk about. Bond length & bond strength. The higher the bond order, the stronger the pull between the. Web bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond length is the average distance between the two nuclei of atoms bonded together in a covalent bond. Experimental > geometry > diatomic bond lengths list of experimental diatomic bond lengths units are: Web the amount of energy required to break all covalent bonds. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Bond length and bond energy. Web bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. The bond length in a h 2 molecule is 74 pm.. Experimental > geometry > diatomic bond lengths list of experimental diatomic bond lengths units are: Web there are charts that reveal the estimated bond length (radii in angstroms or picometers) between two given atoms, according to bond type (i.e. The larger the bond energy, the stronger the covalent bond is. A shorter bond length has higher bond energy. Web in molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. For example, the bond length in a h 2 molecule is 74 pm, as shown in this figure. The bond length is internuclear distance of two covalently bonded atoms. Bond order and length are inversely proportional to each other: Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond). Web bond order and bond length indicate the type and strength of covalent bonds between atoms. Chemical bonding and molecular structure. Web bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Darwent, national standard reference data series, national bureau of standards, no. Test your knowledge with multiple choice flashcards. Bond length to change units) Bond length & bond strength.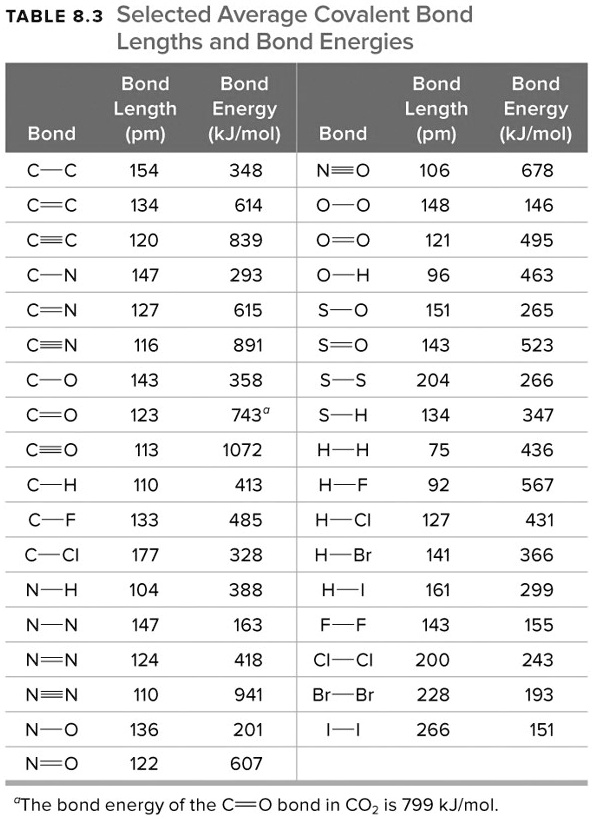
SOLVEDTABLE 8.3 Selected Average Covalent Bond Lengths and Bond

Bond Length and Bond Strength Chemistry Steps

PPT Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint
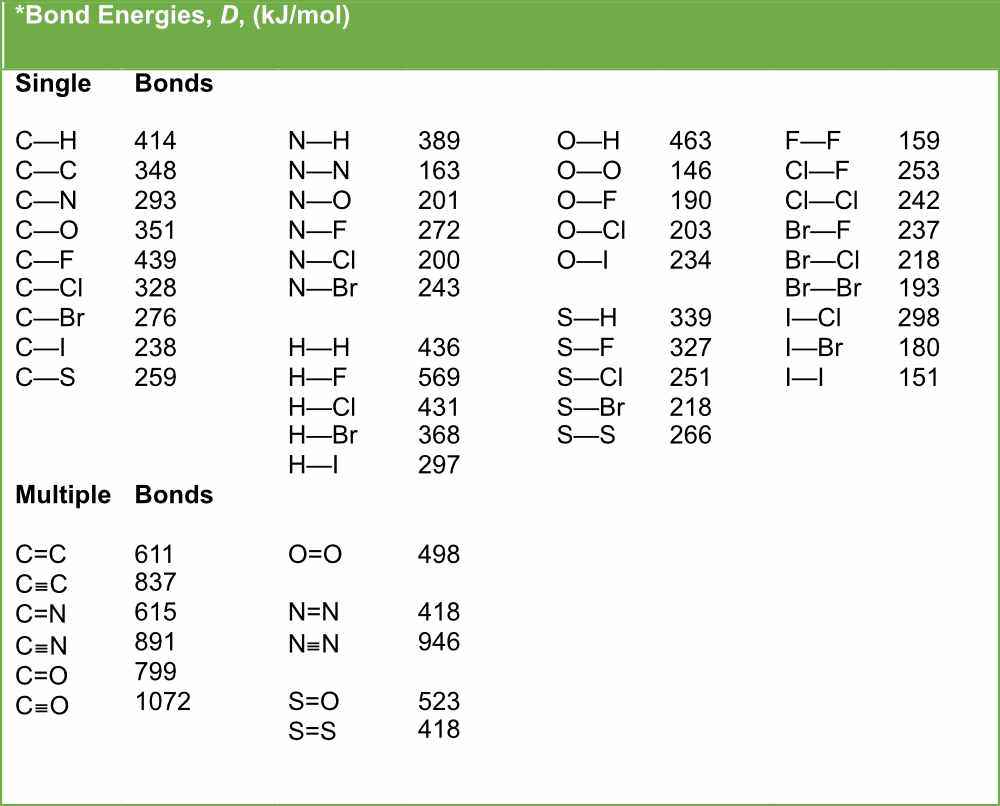
Bond Length and Bond Strength Pathways to Chemistry
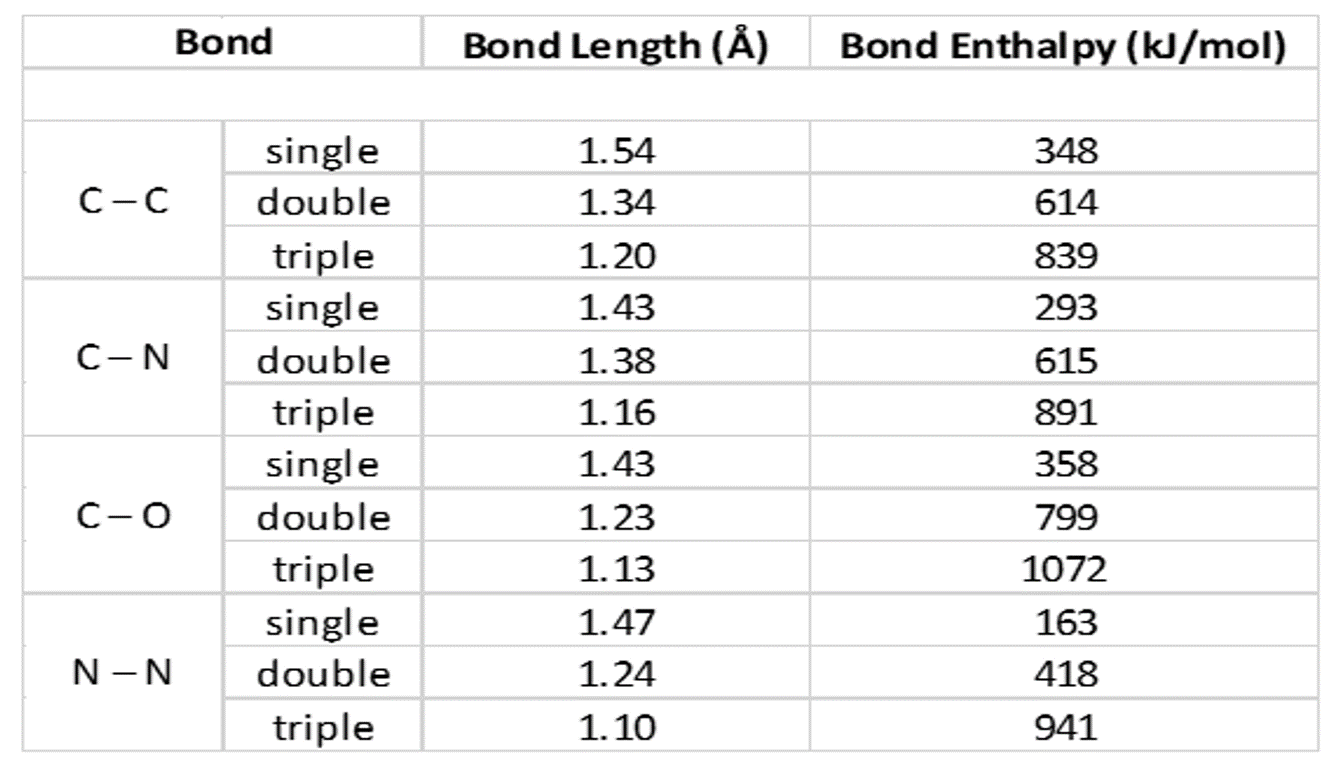
The bond lengths of carboncarbon, carbonnitrogen, carbono Quizlet

Bond Length and Bond Strength Pathways to Chemistry

Individual bondstrengthbondlength parameters for MO bonds in the

Selected bond lengths (Å), bond angles ( • ), and intermolecular

Bond Length Periodic Table

Average Bond Enthalpy Table
Web Bond Length Is Defined As The Distance Between The Centers Of Two Covalently Bonded Atoms.
Bond Energy Is The Potential Energy Required To Break A Covalent Bond.
I Know This Is A Late Response, But From What I Gather We Can Tell What The Bond Order Is By Looking At The Number Of Valence Electrons And How Many Electrons The Atoms Need To Share To Complete Their Outer Shell.
Bond Length And Bond Energy.
Related Post: