Bca Chart Chemistry
Bca Chart Chemistry - The theory behind bca tables (before, change, after) is to create a table underneath the chemical reaction that focuses exclusively on moles. Students only needed to know basic information about acids and bases (acids have a ph < 7, bases have a ph > 7, etc.) to apply it to limiting reactants. The theory behind bca tables (before, change, after) is to create a table below the chemical reaction that focuses exclusion on moles. Web intro to stoichiometry with bca tables. Web this is an intro of how to use a bca table for stoichiometry Web teaching stoichiometry with bca tables. 16k views 8 years ago stoichiometry. One of the biggest advantages in using bca tables over dimensional analysis is an tables produce finish amounts for all chemicals and this lends to very simple limiting reagent solutions. Web one popular method is the “before, change and after” (bca) chart. Web i recently stumbled across a blog about the use of bca (before change after) tables for stoichiometry written by lowell thomson. 16k views 8 years ago stoichiometry. Web this is one introduction to the bca table; The reactants and products, along with their coefficients will appear above. Web a stock bca solution contains the following ingredients in a highly alkaline solution with a ph 11.25: I discuss the method i used with one introductory chemistry class to teach both the algorithm. Web the bca table method of performing stoichiometry calculations that is a cognitive approach that does not rely on algorithms, but rather it engages proportional reasoning skills. Web teaching stoichiometry with bca tables. 16k views 8 years ago stoichiometry. I match using sundry bloggers which teaching bca tables takes commitment and time. I discuss the method i used with one. Web bca tables are shown how to use them and how they connect a balanced reaction as a recipe into stoichiometry calculations. Web a stock bca solution contains the following ingredients in a highly alkaline solution with a ph 11.25: Web an intro to bca tables is shown underneath for those unfamiliar with them. Students only needed to know basic. Web one popular method is the “before, change and after” (bca) chart. I wanted to share my journey, spurred on by my students, into the extensive use of the bca approach in ap and ib chemistry. Make sure you have moles for your starting value. Bicinchoninic acid, sodium carbonate, sodium bicarbonate, sodium tartrate, and copper (ii) sulfate pentahydrate. Melissa incorporates. Web teaching stoichiometry with bca tables. Web these contributions highlighted of technique of by an “before, change, after” (bca) table to organize stoichiometry problems, turning stoichiometry specific include a chemical “sudoku puzzle”. Ice is a simple acronym for the titles of the first column of the table. Web if you can balance chemical equations and convert to moles, you can. We encourage contributions that demonstrate the particular. The remaining values will automatically be calculated. Web a stock bca solution contains the following ingredients in a highly alkaline solution with a ph 11.25: Web this is one introduction to the bca table; 16k views 8 years ago stoichiometry. Insert the starting moles into the bca table and complete the “” row. Web in this blog post i'll describe a recent attempt at using bca tables for teaching stoichiometry. Web an intro to bca tables is shown underneath for those unfamiliar with them. Web bca tables are shown how to use them and how they connect a balanced reaction. Students only needed to know basic information about acids and bases (acids have a ph < 7, bases have a ph > 7, etc.) to apply it to limiting reactants. Web intro to stoichiometry with bca tables. (convert from grams to moles using the molar mass if needed.) step 3: Make sure you have moles for your starting value. We. The reactants and products, along with their coefficients will appear above. Web bca tables are shown how to use them and how they connect a balanced reaction as a recipe into stoichiometry calculations. But once you can use these, you sack get through limiting reagents and then later on in the course, equilibrium, acids, and bases! I stands for initial. Web teaching stoichiometry with bca tables. But once you can use these, you sack get through limiting reagents and then later on in the course, equilibrium, acids, and bases! Web intro to stoichiometry with bca tables. Web bca tables are shown how to use them and how they connect a balanced reaction as a recipe into stoichiometry calculations. Web chemed. Part 2 includes additional practi. Web chemed x invites practitioners in the chemistry education community to share their experiences, knowledge and the resources they use in their classroom and laboratory. I match using sundry bloggers which teaching bca tables takes commitment and time. Web this is an intro of how to use a bca table for stoichiometry Web an intro to bca tables is shown underneath for those unfamiliar with them. I stands for initial concentration. Melissa incorporates modeling into bca tables. The bca assay primarily relies on two reactions. Bicinchoninic acid, sodium carbonate, sodium bicarbonate, sodium tartrate, and copper (ii) sulfate pentahydrate. But once you can use these, you sack get through limiting reagents and then later on in the course, equilibrium, acids, and bases! In a bca, the starting moles, change in moles (determined using the proportions indicated by the balanced equation coefficients), and final moles are recorded in a table. Students only needed to know basic information about acids and bases (acids have a ph < 7, bases have a ph > 7, etc.) to apply it to limiting reactants. Web if you can balance chemical equations and convert to moles, you can use this hack to correctly so.more. If you struggle with stoichiometry problems in your chemistry class, the method. I was thrilled to discover chemed xchange! Ice is a simple acronym for the titles of the first column of the table.
BCA Table Stoichiometry (Mole Mass) YouTube

Using Visual BCA Tables to Teach Limiting Reactants Chemical

Intro to Stoichiometry with BCA Tables YouTube

PPT The BCA Method in Stoichiometry PowerPoint Presentation, free
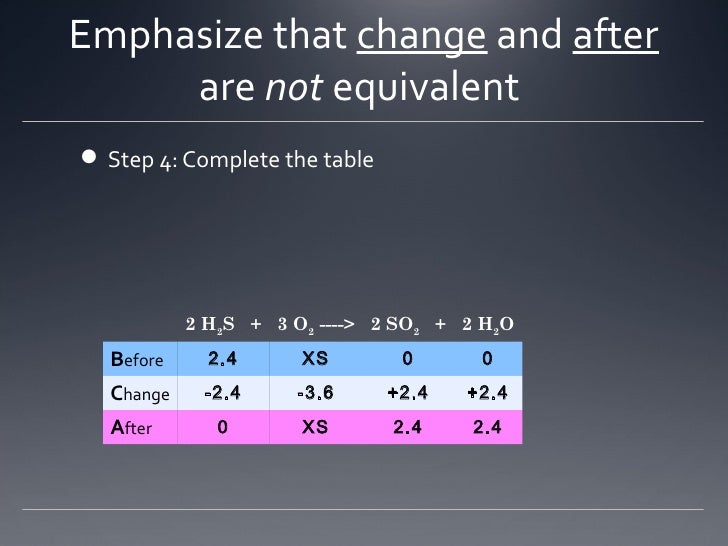
Before, Change, After (BCA) Tables for Stoichiometry
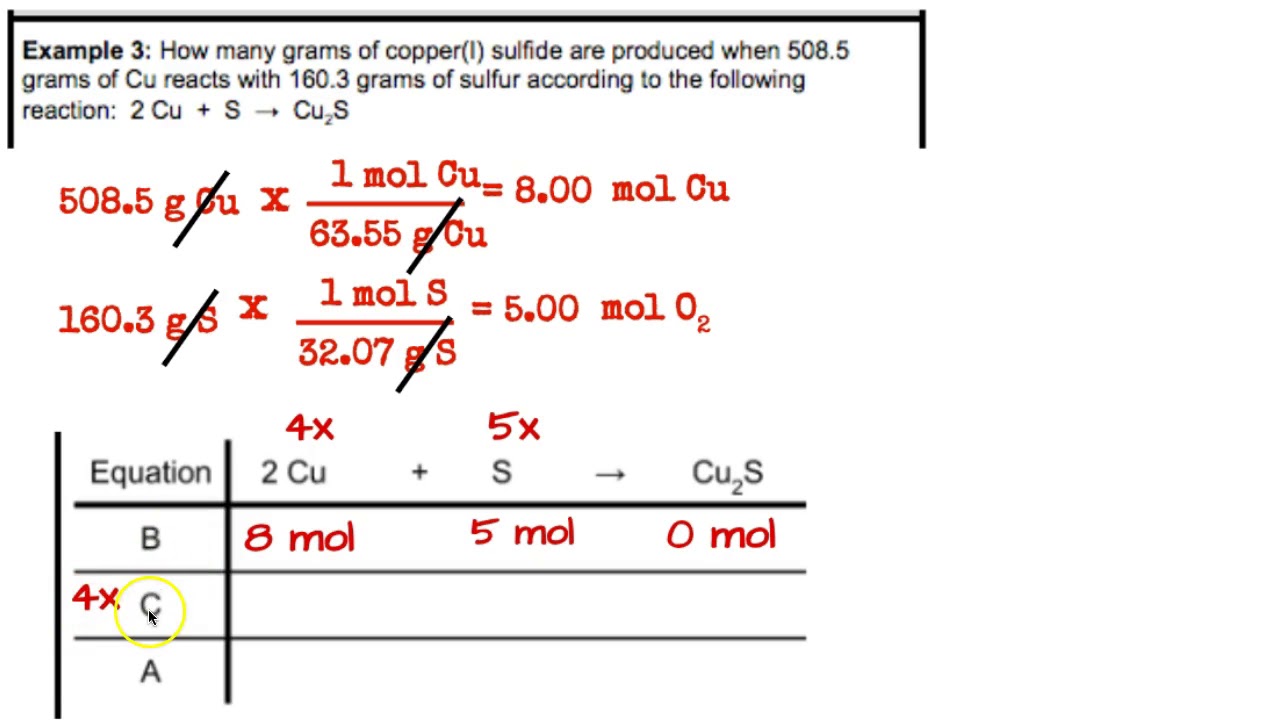
Molar Mass & BCA Tables Examples 3 & 4 YouTube
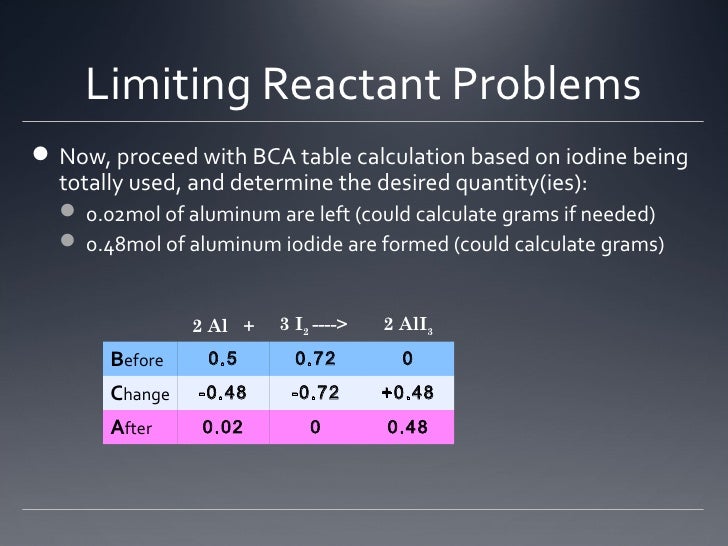
Before, Change, After (BCA) Tables for Stoichiometry

Intro to BCA tables YouTube

Limiting Reactants and BCA Tables YouTube

BCA Tables and Moles YouTube
Web Ice Tables Automatically Set Up And Organize The Variables And Constants Needed When Calculating The Unknown.
This Is Probably The Greatest Useful Yet One Of The More Annoying Parts Of An Chemistry Curriculum.
This Row Contains The Initial Concentrations Of Products And Reactants.
Web This Is One Introduction To The Bca Table;
Related Post: